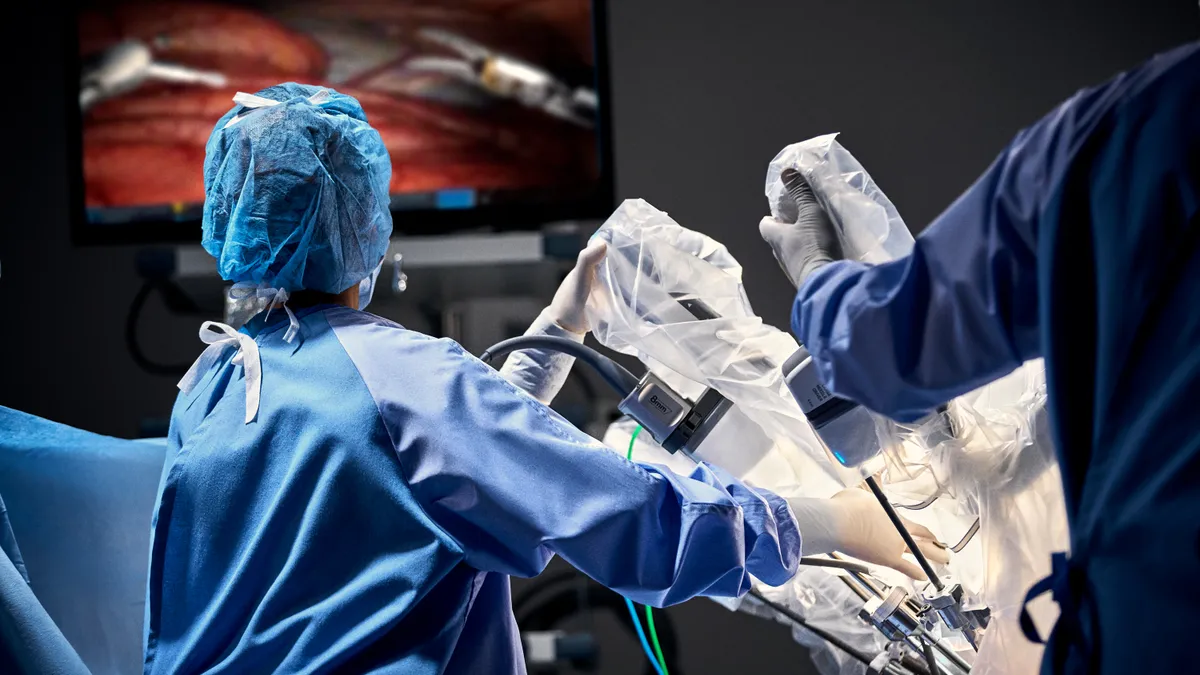
Dive Brief:
-
FDA has cleared two Intuitive technologies for use in sealing procedures, the Sunnyvale, California-based company said last week.
-
The devices add to the capabilities of Intuitive’s da Vinci X and Xi, robotic systems that make up a growing portion of the company's base of installed products.
-
One of the devices, SynchroSeal, is used to transect tissue. The other product, the E-100 generator, provides power to SynchroSeal.
Dive Insight:
Intuitive has established a source of revenues beyond large, one-off sales of robotic surgical systems by introducing a range of instruments and accessories. Last year, surgeons used between $700 and $3,500 worth of Intuitive instruments and accessories per procedure. Those procedures generated revenues of $2 billion, almost twice as much as Intuitive made from sales of da Vinci systems.
Combined with the $635 million in service revenues, demand for instruments and accessories resulted in 71% of Intuitive’s turnover last year coming from recurring sales. By continuing to add new instruments, Intuitive is working to grow recurring revenues and hold off mounting competition.
SynchroSeal is the latest instrument to receive FDA clearance. According to Intuitive, "SynchroSeal will enable a surgeon to perform rapid one-step sealing and transection with a single pedal press." The technology is designed to enable surgeons to perform these actions more quickly than they can using Vessel Sealer Extend on the integrated da Vinci X and Xi Erbe VIO dV generator.
Both SynchroSeal and Vessel Sealer Extend stand to benefit from the other device recently cleared by FDA. The E-100 generator upgrades the power to the two sealing instruments. Intuitive described the product as "its first internally developed robotic generator."
The E-100 and SynchroSeal clearances bring the number of Intuitive devices cleared by FDA this year up to 10. Last year, FDA cleared 13 Intuitive devices, down slightly on the 16 products the agency green lit in 2017.
Intuitive’s 2017 crop of clearances included its flagship da Vinci Xi system and its stripped-down, lower-cost sibling, da Vinci X. Since then, da Vinci has introduced instruments and accessories for use with da Vinci X and Xi, giving users of its older robotic surgical systems more reasons to upgrade.
In the third quarter, 42% of system placements involved trade-ins. Talking to investors last month, Intuitive CFO Marshall Mohr attributed the level of trade-in activity to "customer desire to access or standardize on our fourth generation technology."