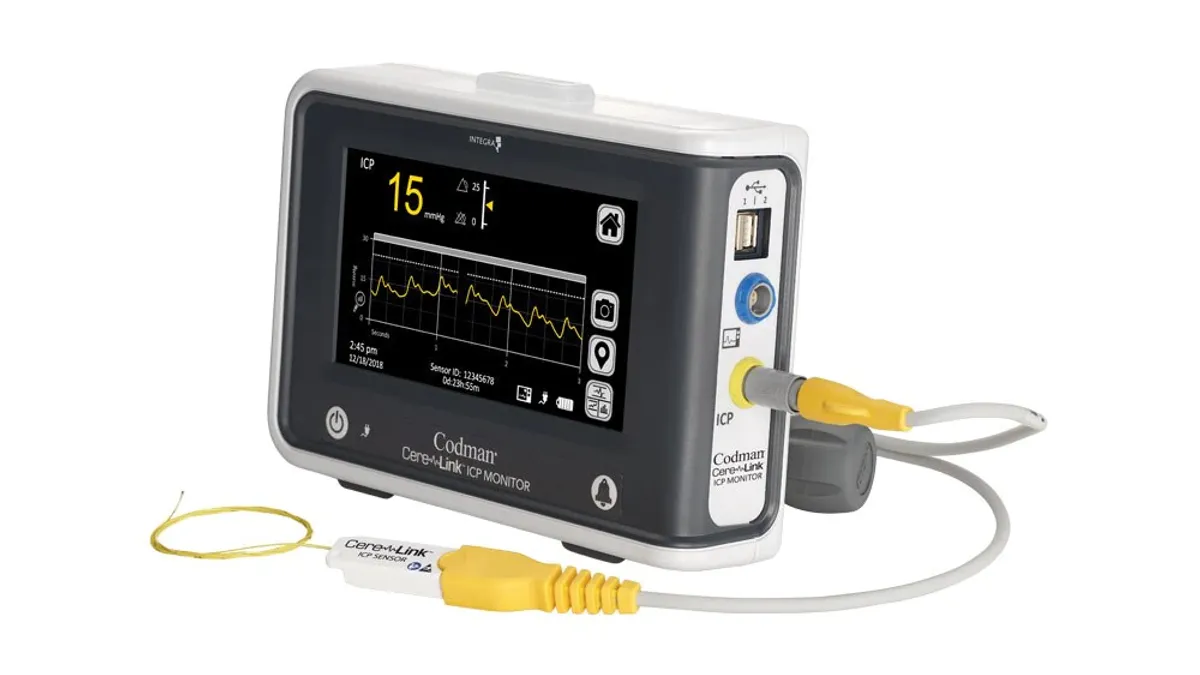
Integra LifeSciences announced an immediate global voluntary removal of its CereLink intracranial pressure monitors, after receiving reports from some customers that their readings were out of range. The Princeton, N.J.-based medical device manufacturer is investigating the problem, and plans to resume shipment of the monitors as soon as the problems have been resolved, Integra said in a Wednesday morning filing with the U.S. Securities and Exchange Commission.
The out-of-range readings are primarily caused by electrical interference from the external environment or from a component on the circuit board of the monitor, according to the filing. So far, the incorrect readings have happened at a low incidence rate and at a limited number of sites, the company stated, but Integra decided to remove all CereLink monitors from the field “out of an abundance of caution.”
The company expects a $9 million returns provision in the third quarter, and lowered its sales forecast accordingly. It now expects revenue of $374 million to $382 million in the third quarter.
“While [management] does expect the recall to have some impact on CereLink sales, this shortfall will be absorbed in the back half of the year by incremental upside from the balance of the business,” J.P. Morgan analysts wrote in a Wednesday research note.
In June, the company issued a field correction to customers informing them about the problem and troubleshooting techniques while it investigated the root cause, according to an FDA record. The recall included 1,210 devices worldwide. The FDA classified it as a Class I recall, its highest risk category, in July.