Dive Brief:
- Insulet issued an urgent medical device correction on Monday related to battery problems with a component of its Omnipod DASH system.
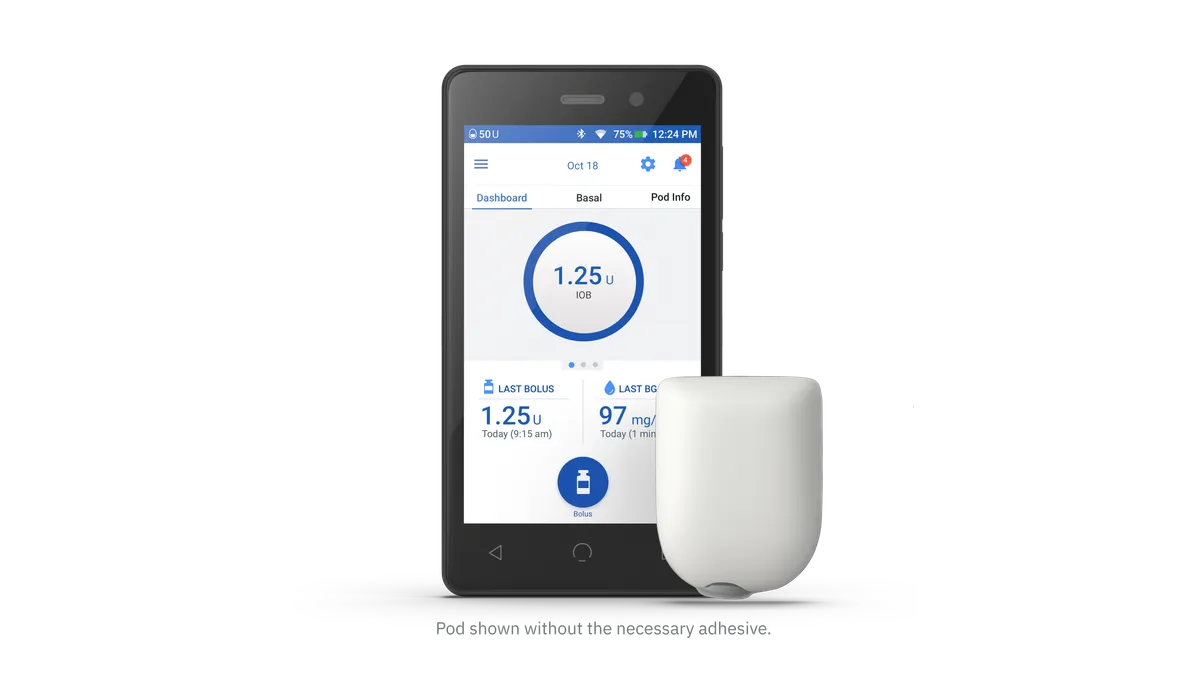
- The device uses a wearable insulin pod that’s controlled by a personal diabetes manager (PDM), a smartphone-like device that does the calculations for bolus insulin doses.
- Insulet plans to replace the PDMs for all of its current Omnipod DASH users globally, incurring an aggregate charge of $35 million to $45 million, J.P. Morgan Analyst Robbie Marcus wrote in a Monday research note.
Dive Insight:
Insulet said it received reports of some Omnipod DASH users having battery problems with their PDM devices, including the battery swelling, fluid leaking from the battery, and in rare cases, extreme overheating. In a letter to users, the company said it plans to ship updated devices to all current Omnipod DASH customers in the coming months.
The battery issue applies to all of Insulet’s Omnipod DASH PDMs, but the likelihood of problems may increase if the device has been in use longer than 18 months. Charging the device to a full battery and leaving it on the charger overnight also increases the risk.
So far, Insulet said it has not received reports of any injuries related to the battery issues.
The company advised patients to monitor their PDMs for battery problems, including a bulging back cover and the device losing its charge very quickly, overheating or emitting an odor.
If patients notice any of these problems, they should not charge the device, stop using the system and switch to a backup insulin plan as soon as they can. Users can also contact Insulet for a temporary replacement device.
Insulet’s management had called out higher warranty costs associated with the Omnipod DASH PDMs in its second quarter earnings call.
“Given the clarity on the root cause of the issues, management expects a software fix that limits charging beyond a certain threshold to address the problem,” J.P. Morgan’s Marcus wrote.
Most of the $35 million to $45 million in costs from replacing the devices is expected to affect the cost of revenue in the third quarter and operating expenses in the fourth quarter and 2023, he added.