Dive Brief:
-
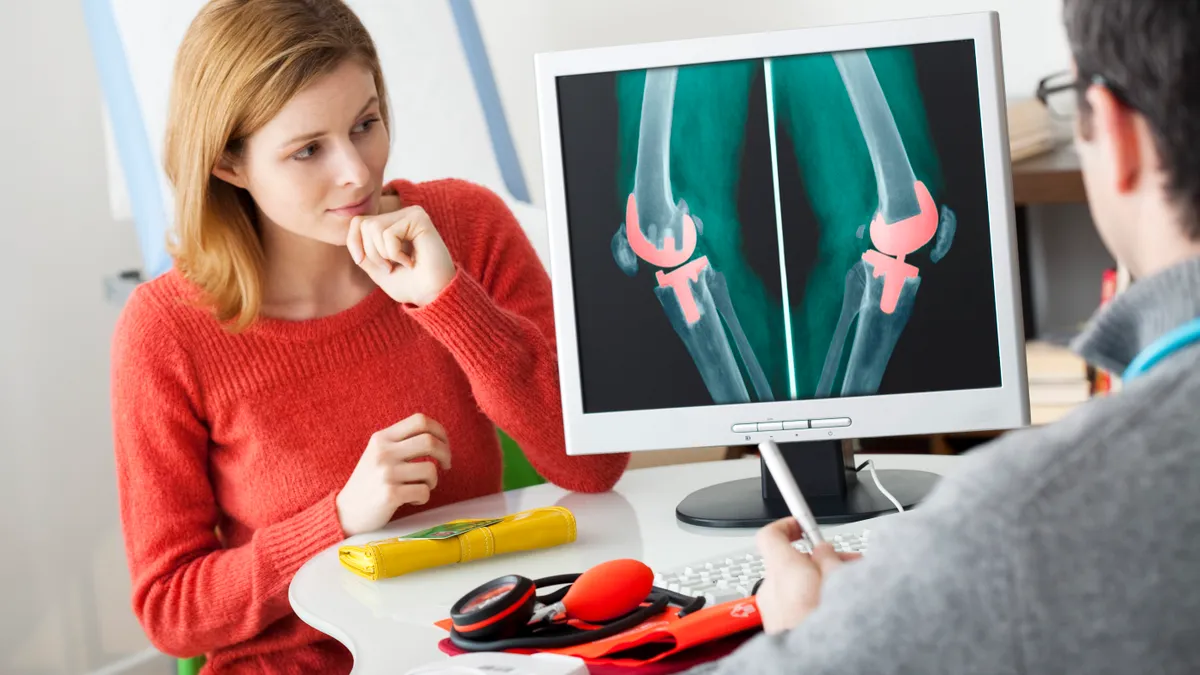
FDA has awarded breakthrough device status to Garwood Medical Devices’ technology for tackling biofilm infections on prosthetic knee implants, the company said Tuesday.
-
Garwood, working with technology developed by the University at Buffalo, thinks it can cut the rate of joint replacement infections, thereby reducing the need for follow-up surgeries.
-
The device uses electrical stimulation to disrupt the bacterial biofilms that make it hard to treat infections using antibiotics.
Dive Insight:
Biofilms protect bacteria from the immune system and antibiotics. When such a biofilm forms on the surface of a prosthetic implant, it can lead to periprosthetic joint infection (PJI), a complication that causes more than 25% of revisions to arthroplasty procedures. The revision procedure is associated with a high mortality rate and significantly adds to the cost of treating patients.
Mark Ehrensberger and his colleagues at the University at Buffalo set out to tackle PJIs by creating a way to prevent and eradicate biofilms. The research project resulted in a device that uses electrodes to modulate electrochemical processes on the surface of metallic prosthetic implants.
Garwood licensed the technology, now known as BioPrax, and sought to build on preclinical evidence of its effectiveness generated by Ehrensberger and his colleagues. The preclinical studies run by the Buffalo researchers showed using the device on titanium implants for one hour reduces the presence of bacterial colony forming units but that the antimicrobial effects last less than one week.
Using the device in combination with the antibiotic vancomycin produced better results. A one-week course of the antibiotic yielded a 99.8% drop in bacterial colony forming units. However, as bacteria were still present the researchers tried a five-week course, bringing the presence of microorganisms down to undetectable levels.
Those studies used rodent models but generated encouraging enough data to attract Garwood and its investors. Having raised a $3.6 million Series A financing round in 2016, Garwood expects to raise $3 million in Series B funding by the end of this year.
The money and breakthrough status will support Garwood’s efforts to bring BioPrax to market. As part of the breakthrough program, Garwood stands to benefit from closer interactions with the FDA and an expedited review if it makes it the filing stage.