Patients, physicians and healthcare officials are facing a new “reality” in treatment and care that’s spurring questions among the medical community about safety and oversight.
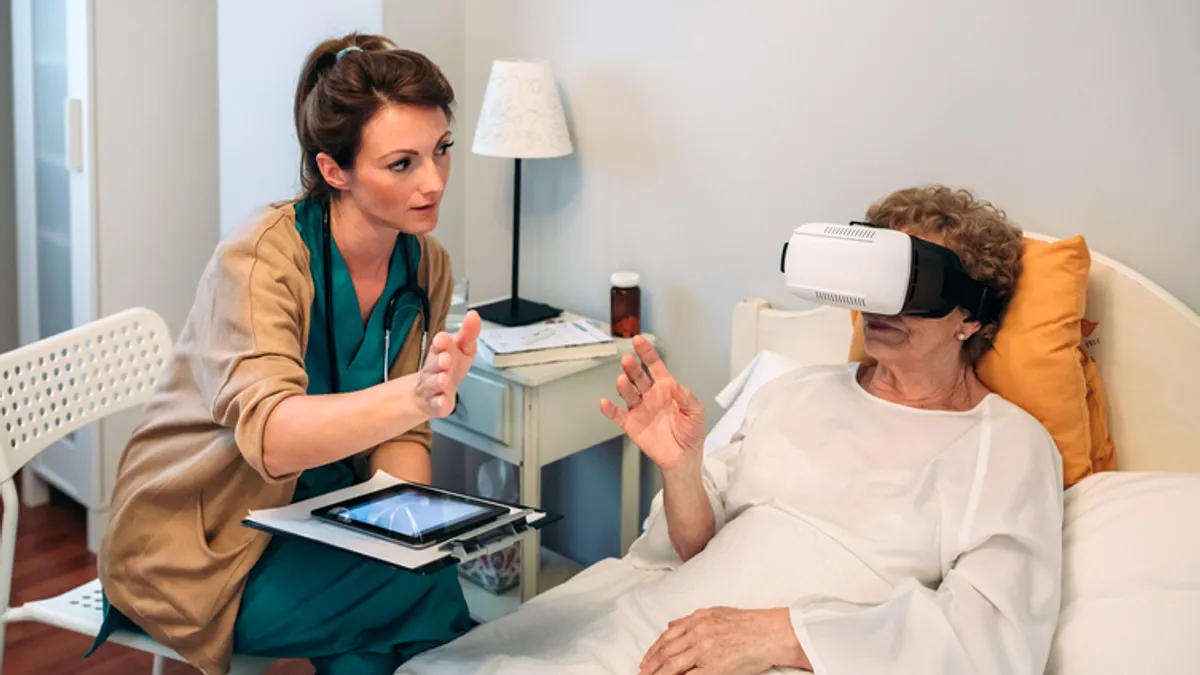
That’s because virtual- and augmented-reality headsets, once thought of as devices just for gaming, now are being used in operating rooms, for physician training and for in-home treatments.
Last year, Johns Hopkins performed its first surgery using augmented reality to show medical scans and data above a surgical site. The Food and Drug Administration also cleared the first VR-based treatment intended to reduce chronic back pain.
As more of these headsets and accompanying software tools are used in patient care, regulators are grappling with questions such as how to separate out devices that blend clinical software with commercial headsets, and how to best ensure the devices are safe and effective.
It also sparked the FDA to hold a meeting last week where patients and caregivers provided suggestions for how these devices should be regulated in the future.
In the July 12 and 13 meeting, a patient engagement advisory committee talked about what informed consent would look like in a surgery that used a supplemental augmented reality tool, a key topic for future regulation.
“I think the surgeon would have to be able to explain the surgery in such a way to say, ‘this is what I'm going to do. This is how I'm going to do it.’ And if there's a possibility of showing the patient what the augmented reality objects are going to do, that would be great,” said Colleen Gallagher, a committee member and executive director of clinical ethics at MD Anderson Cancer Center in Houston.
Gallagher added that surgeons should be able to describe their team’s education in using AR tools, and patients should be able to choose whether these tools are used in their surgery.
Committee members also brought up questions about how the devices would be regulated, especially when it comes to software changes that may change their performance. The FDA has floated the idea of handling changes in software through a predetermined change control plan, where developers describe the scope of changes they expect before a product is cleared, but hasn’t issued any guidance yet.
“As the technology changes, the software changes, what does that look like? What is the training regimen associated with that and who is doing the oversight? And what are the checks and balances associated with that? I think that there probably needs to be some regulation around that,” said Philip Rutherford, a committee member and chief operating officer of the patient advocacy group Faces & Voices of Recovery.
That regulatory process also includes questions about equity, such as what patients will have access to these technologies, and how will patients know that it works on people that look like them?
In cases where the devices are used as home treatments, the committee discussed how to differentiate between “consumer” uses of VR headsets, such as gaming, versus clinical uses, such as performing daily physical therapy or mindfulness activities.
“I would say that it's important to distinguish the medical use side versus the entertainment side,” said Amye Leong, a committee member and CEO of Healthy Motivation. “When [patients] insert that medical software, do they know there's a particular goal that they have to achieve?”
Finally, patients should be able to easily report problems with the headset or software, including adverse events, the committee said. They suggested creating a registry of devices so that patients and healthcare providers can easily view any safety notices.
How does that work when prescription software is combined with a consumer headset, which isn’t regulated by the FDA? The agency said for these types of “dual use” devices, it can coordinate with other government agencies, such as the Consumer Product Safety Commission or the Federal Trade Commission.