Dive Brief:
- Medtronic said Tuesday it received FDA premarket approval for its Micra AV pacemaker, a device it expects to more than triple the potential market for its leadless pacing technology.
- The latest version of the product, a key talking point in the company’s cardiovascular pipeline that builds on a 2016 PMA, is an implant designed to treat atrioventricular or AV block, a condition where the electrical signals between the heart’s upper and lower chambers are out of sync.
- Medtronic supported the approval with data from the 75-patient MARVEL 2 study, in which the device demonstrated a 63% reduction in major complications compared to traditional pacemakers.
Dive Insight:
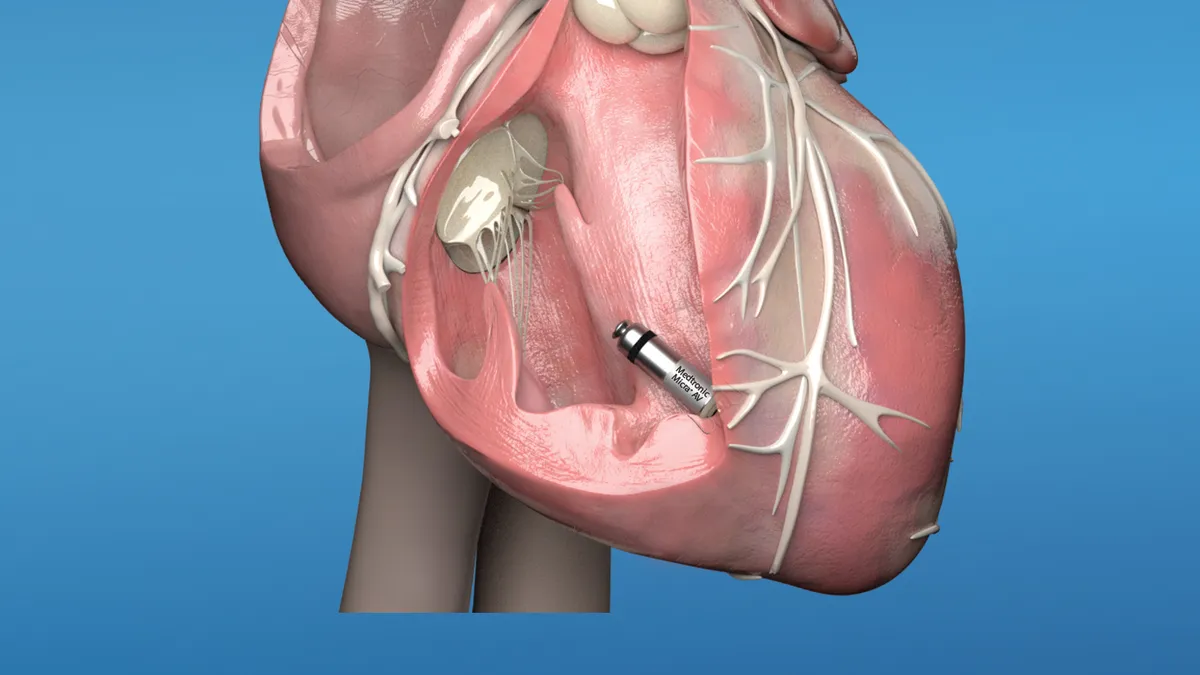
Patients with AV block have had the option to be treated with traditional dual-chamber pacemakers implanted beneath the collar bone and attached to the heart with thin wires called leads. The updated Micra has internal sensing algorithms that detect atrial contractions and adjust pacing in the ventricle to coordinate with the atrium in patients with AV block.
Medtronic received FDA approval for its first Micra pacemaker in 2016. The miniaturized pacemaker is delivered to the heart in a transcatheter approach through a vein in the leg and attached directly to the right ventricle with four tines. The leadless technology is intended to avoid complications such as lead fracture and generator pocket infection that can occur with traditional pacemakers.
The original Micra transcatheter pacing system has been used to treat patients needing a single chamber pacemaker. Approval of the Micra AV expands the leadless option to patients with heart block who otherwise would have been treated with dual chamber pacing.
At the J.P. Morgan Healthcare Conference last week, retiring Medtronic CEO Omar Ishrak touted the product as one he's most excited about. The company said it expects the AV version of the device to more than triple the target population for the leadless system, from about 15% to about 55% of pacemaker patients. In the latest quarter, Micra generated "low 20s" U.S. revenue growth for the company.
Medtronic said it will begin training field personnel and physicians, activating a limited number of implanting centers in the coming weeks, and expects a full launch of the pacemaker later this spring.
St. Jude Medical halted implants of a competing leadless pacemaker, the Nanostim, in Europe in 2016 after receiving reports of premature battery failure. After Abbott acquired St. Jude, it alerted doctors in 2017 to an additional problem with a component used in retrieval procedures for the device.