FDA took a step in its evolving regulation of artificial intelligence-assisted radiological imaging Friday, announcing a green light for an AI-based software meant to help less specialized medical professionals such as registered nurses take cardiac ultrasound images in adult patients.
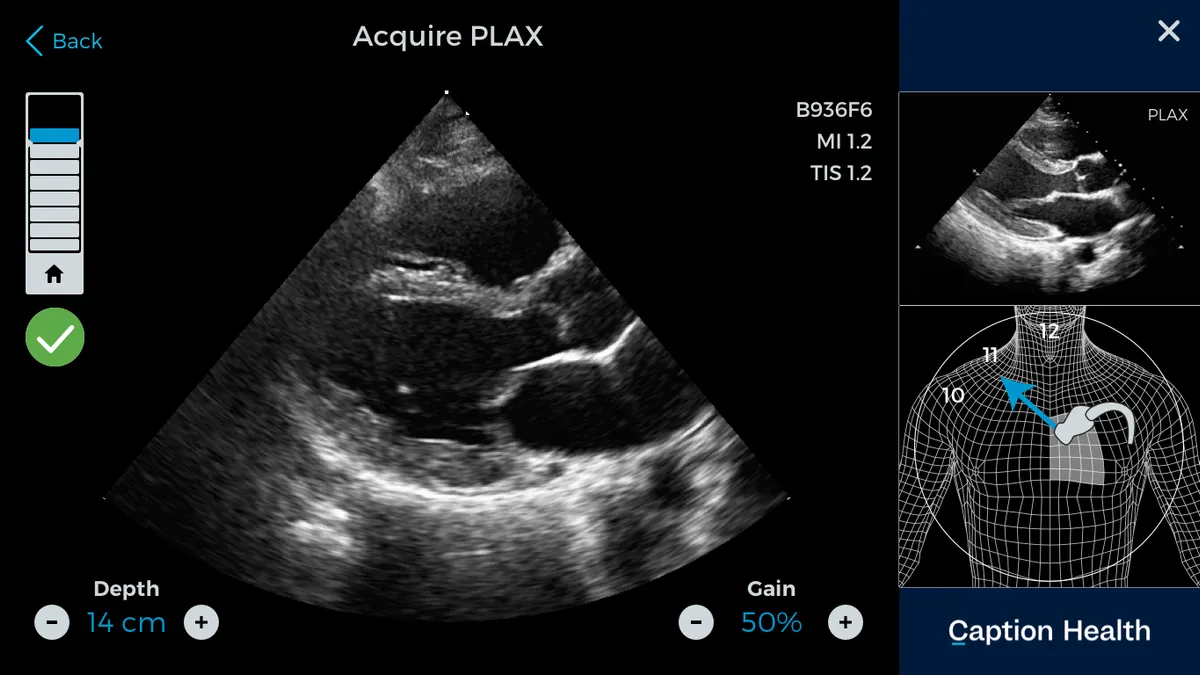
Designated as a breakthrough device, San Francisco-area startup Caption Health touts the product as "a co-pilot for clinicians" that gives guidance on how to best handle a transducer and real-time feedback on the quality of an image.
Those pictures and videos, also known as echocardiograms, are used to diagnose a range of heart conditions. FDA said a cardiologist is still supposed to do a final review of any images as part of patient evaluation.
Caption said in its announcement that the De Novo authorization comes amid "democratization" of ultrasound thanks to increasing portability and affordability of the technology, but noted that difficulty in getting a good image has prevented wider adoption.
The authorization creates a precedent for follow-on 510(k)s in the "Image Acquisition Guided By Artificial Intelligence" category. "This device type is fairly broad, intentionally so on FDA's part," Sam Surette, a former FDA reviewer and Caption's head of regulatory affairs and quality assurance, told MedTech Dive.
Robert Ochs, deputy director of FDA's office of IVDs and radiological health, heralded the go-ahead as "especially important because it demonstrates the potential for artificial intelligence and machine learning technologies to increase access to safe and effective cardiac diagnostics that can be life-saving for patients.”
The decision was supported by a prospective trial assessing how effectively eight registered nurses, with no prior ultrasound experience but given a short training, were able to perform 10-view exams on 240 patients. Five cardiologists then assessed whether exam quality was good enough to make qualitative visual assessments of left ventricular size and function, right ventricular size and fluid around the heart, finding the software helped nurses meet all primary endpoints.
Another study found 50 trained sonographers were able to capture comparable diagnostic images with and without the software's help.
FDA's review of the technology took a little more than five months.
The OK comes just weeks before FDA will hold a two-day workshop on AI in radiological imaging. Last spring, the agency shed some light on its thinking in a white paper on modifications to AI and machine learning-based software as a medical device. But across the board, FDA's approach to regulating relatively nascent AI remains a work in progress.
The meeting will address Caption's area of guided image acquisition, as well as AI devices mean to automate diagnostic radiology workflow.
"The FDA and industry [are] very familiar with how to validate diagnostic, machine learning and deep learning tools," Surette said.
"What is interesting ... is how do you both verify and validate the performance of an algorithm that operates in real time alongside a user? ... I think a lot of the discussion will be around that topic of the user type. How do you show that the user is becoming effective, or more effective than they were without the guidance or tool?"
The Caption Guidance software can currently only be used with a specific FDA-cleared diagnostic ultrasound system produced by Teratech Corporation, but has potential to be expanded to other ultrasound imaging systems with the right technical specifications.
Caption Guidance received the agency's breakthrough device designation in September 2018, shortly after the company gained 510(k) clearance for automated ejection fraction interpretation software. The company said it plans to market the two products together as Caption AI.
Up until October, Caption did business as Bay Labs. CEO Andy Page joined the company a year ago and is a former president at Livongo and 23andMe. The company counts Minneapolis Heart Institute, Khosla Ventures and Edwards Lifesciences among its investors.
It announced a partnership with Edwards in 2018 focused on heart disease detection. Ultrasounds can be used to diagnose severe aortic stenosis, the condition Edwards targets with its transcatheter aortic valve replacement technologies.
According to FDA's database, the product is the first De Novo clearance awarded this year. The agency greenlighted 22 devices through the pathway in 2019, half the 44 authorized the prior year.