Dive Brief:
- The Food and Drug Administration said Friday it has authorized the first home test for the sexually transmitted infections chlamydia, gonorrhea and trichomoniasis.
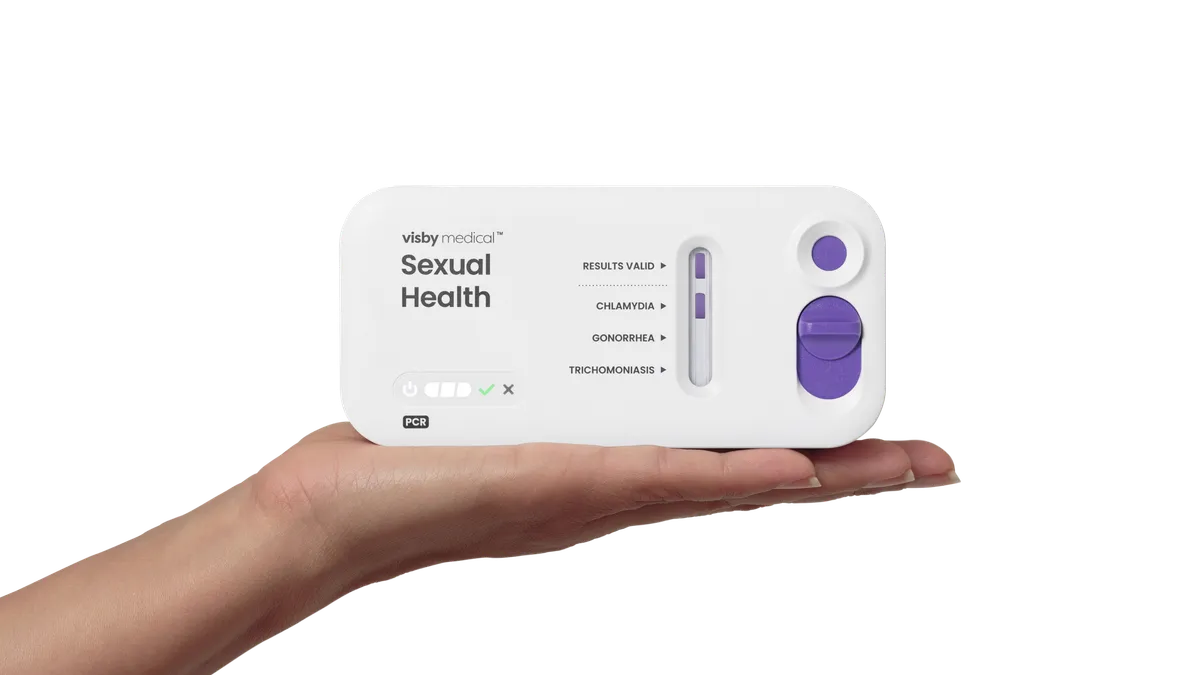
- Visby Medical received authorization for the single-use, polymerase chain reaction test via the de novo pathway. Women self-collect vaginal swabs and analyze them using a powered testing device. The diagnostic can be purchased without a prescription.
- The FDA established special controls as part of the de novo authorization, creating a route for other companies to bring similar tests to market via the 510(k) clearance pathway.
Dive Insight:
Visby operated under the radar for the first seven years of its history, only breaking cover when it won emergency use authorization for a COVID-19 test in 2020. By then, the company had already completed enrollment of 1,585 participants in a study of its sexual health test.
Visby went on to raise $135 million in 2022, and received 510(k) clearances for its first- and second-generation sexual health tests.
The clearances and associated waivers under the Clinical Laboratory Improvement Amendments of 1988 allowed healthcare professionals to use the tests in healthcare settings. In those settings, the tests eliminate the need to send samples to labs and allow physicians to diagnose and treat the infections in a single patient visit.
Visby’s de novo authorization supports home use of the test. Courtney Lias, director of the Office of In Vitro Diagnostic Devices at the FDA, said in a statement that home testing “can be particularly important for sexual health tests for which patients may experience fear or anxiety, possibly resulting in delayed diagnosis or treatment.”
The FDA granted the authorization after Visby showed the test correctly identified almost all negative and positive samples from women with and without symptoms. The test correctly identified 97.2% of positive Chlamydia trachomatis samples. All the other results were higher, with the figures rising to 100% for the correct identification of positive Neisseria gonorrhoeae samples.
Visby’s authorization continues the expansion of the range of at-home sexual health tests that are available in the U.S. The FDA authorized the first test for chlamydia and gonorrhea with at-home sample collection in 2023 and the first at-home syphilis test in 2024.
More than 2.4 million cases of syphilis, gonorrhea and chlamydia were diagnosed and reported in the U.S. in 2023, according to the Centers for Disease Control and Prevention. Trichomoniasis, which is caused by a protozoan parasite, is estimated to be the most prevalent nonviral sexually transmitted infection globally and affects 2.6 million people in the U.S.