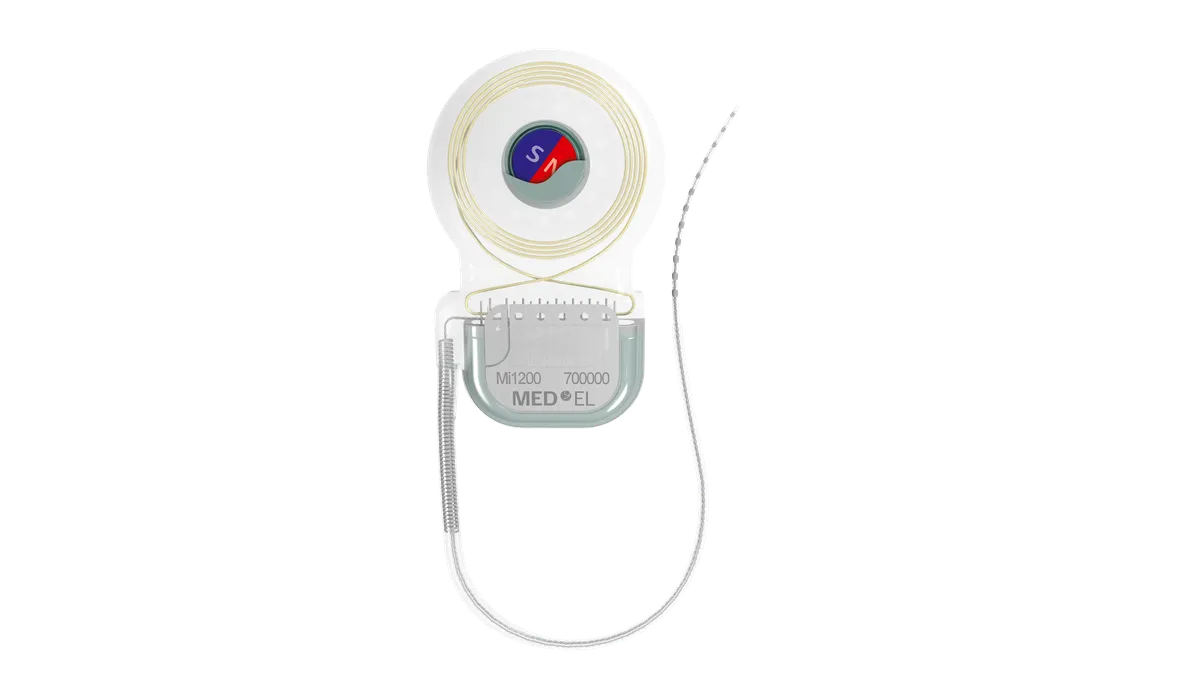
Dive Brief:
- Hearing device maker MED-EL said it received FDA’s first-ever approval for a cochlear implant system for asymmetric hearing loss and single-sided deafness.
- With both conditions, people have difficulty hearing, particularly in noisy environments. The FDA indications were based on a study at the University of North Carolina at Chapel Hill that evaluated speech perception in quiet and noise, sound localization and quality of life in 40 participants age 18 and older.
- No changes to the approved devices in the MED-EL cochlear implant system are required for the new indications, the Innsbruck, Austria-based company said.
Dive Insight:
Nearly 5 million U.S. adults experience single-sided deafness, estimates suggest. Yet hearing aid use in this population is relatively low, a national epidemiologic study found. The condition can negatively impact social interactions, sometimes affecting speech and language development.
The hearing difficulty can stem from head shadowing sounds on the opposite side of the hearing ear and the inability to localize sounds. The condition can be caused by a viral infection, Meniere’s disease or trauma to the ear, and sometimes there is no known cause. Options for patients such as hearing aids and bone conduction devices reroute the signal to the hearing ear and do not restore sound localization or spatial hearing.
Cochlear implants work by electrically stimulating nerves inside the inner ear. MED-EL said its implants are indicated for patients age 5 and older with single-sided deafness and profound sensorineural hearing loss in one ear and normal hearing or mild loss in the other ear.
The devices are also indicated for those with asymmetric hearing loss who have profound sensorineural hearing loss in one ear and mild to moderately severe loss in the other ear, with a difference of at least 15 dB in pure tone averages between ears. Individuals must have had limited benefit from a unilateral hearing aid, defined by hearing word test scores of 5% correct or less in the ear to be implanted alone.
In the clinical study at the University of North Carolina at Chapel Hill, participants with both forms of hearing loss improved their ability to understand speech after one year of implant use when tested with the implant alone. For the people with single-sided deafness, average scores on repeating single words in quiet increased from 4% before surgery to 55% after 12 months with the implant. People with asymmetric hearing loss saw scores improve from 6% to 56%.