Dive Brief:
- The Food and Drug Administration granted de novo clearance to an AI tool to help clinicians predict and diagnose sepsis, the first time the agency has authorized such a tool.
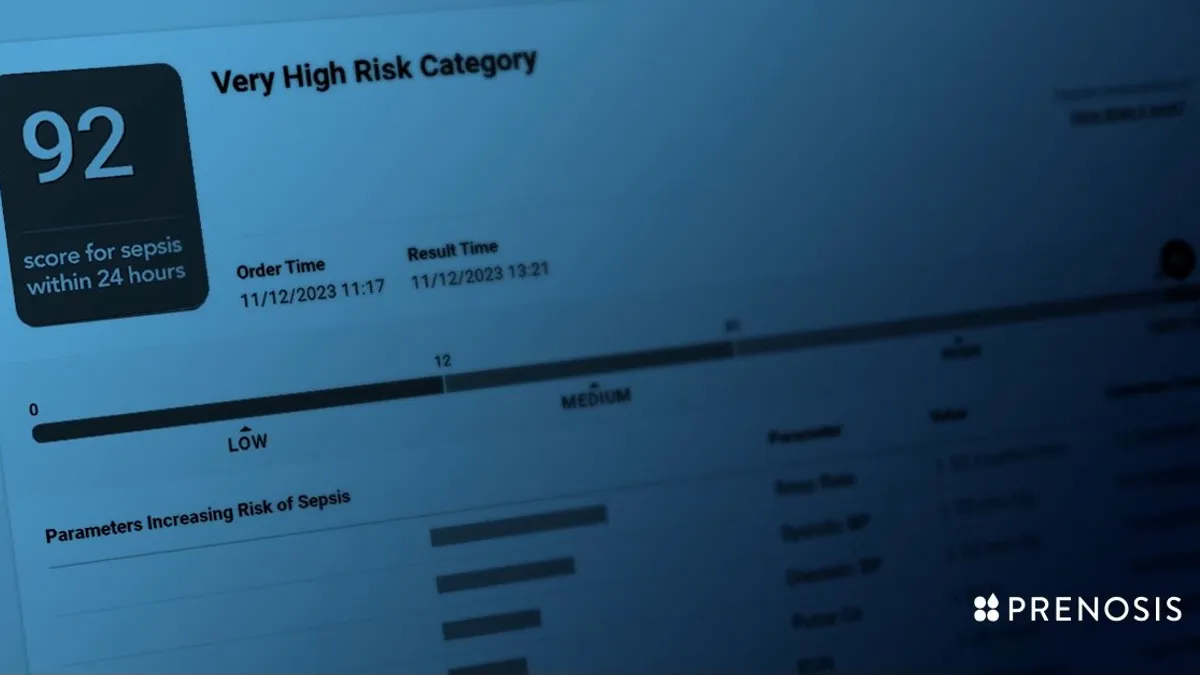
- The Sepsis Immunoscore software, developed by Chicago-based Prenosis, provides a risk score for clinicians on a patient having or developing sepsis within 24 hours. The score is based on 22 parameters, including respiratory rate, blood pressure and white blood cell count.
- Hospitals already use early sepsis detection tools, despite lacking FDA review. The agency clarified in a final guidance in 2022 that clinical decision support software that provides a risk score or probability of a condition should be regulated as a medical device.
Dive Insight:
Sepsis is a severe response to an infection and is responsible for nearly 270,000 deaths each year. It requires prompt treatment, but symptoms can vary across different people.
Because it is such a big problem, software companies and hospitals have dedicated resources to building early detection tools that can alert clinicians when a patient may be at risk of developing sepsis. These tools have recently come under scrutiny after researchers published a study in 2021 finding a widely used tool developed by electronic health record company Epic Systems only correctly predicted the risk of sepsis 63% of the time before clinician intervention.
Prenosis CEO Bobby Reddy Jr. said the company decided to take a different approach, seeking FDA clearance before the agency set out that requirement. The company received de novo authorization on April 2, which will allow its software to serve as a predicate for other sepsis tools.
Reddy pointed to a culture clash in the space with some companies breaking the rules by marketing their AI products without authorization.
“You have companies that are really used to regulation and rely on trust,” Reddy said in an interview with MedTech Dive, adding that some firms are excited about AI, but don’t have as much of an understanding or culture around regulations.
“We don’t think it’s right to have this out on the market without third-party validation,” the CEO added.
The Sepsis Immunoscore has four risk categories: a patient’s rate of sepsis within 24 hours, in-hospital mortality, length of stay in the hospital and ICU admission in 24 hours. The tool is integrated into the electronic health record and displays a number from 0-100 to indicate the risk of sepsis. It also provides a list of all 22 parameters, ranking them in the order of which contributed the most to the increased risk for that patient.
“A lot of clinicians don’t trust AI products for multiple reasons,” Reddy said. “We are trying very hard to counter that skepticism by making a tool that was validated by the FDA first, and then the second piece is we’re not trying to replace the clinician.”
The tool was cleared based on a 750-person study across three sites. Prenosis ran sub-analyses to assess the tool’s performance across gender, race and health comorbidities.
Roche Diagnostics started collaborating with the company in 2020 to expand Prenosis’ core dataset and help it work toward FDA clearance.