Dive Brief:
-
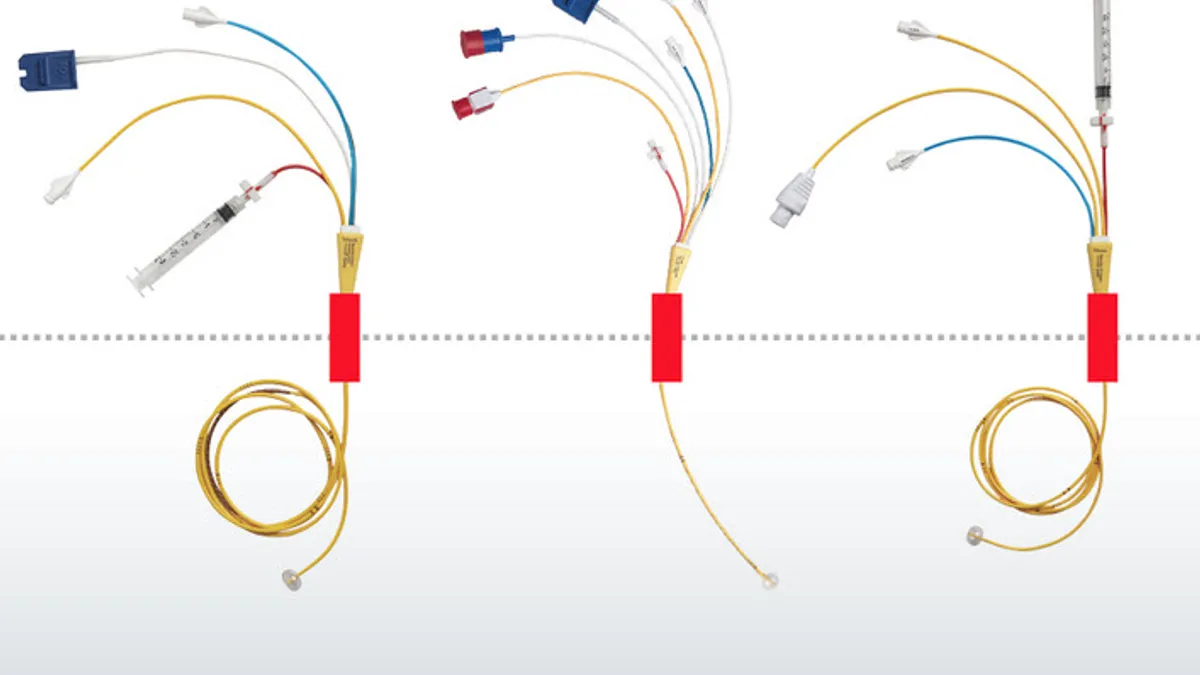
FDA has classed Edwards Lifesciences' recall of its Swan-Ganz Thermodilution Catheter as a Class I event.
-
Edwards began recalling 1,426 of the diagnostic devices late last year after learning that an assembly error caused some products to provide inaccurate pulmonary artery and central venous pressure values and waveforms.
-
While Edwards has not learned of any complications arising from the fault, the potential for the inaccurate information to lead to serious harm or death led FDA to categorize the event as the most serious type of recall.
Dive Insight:
The problem stems from the assembly of the devices. As the catheter tubes on some of the devices are reversed, the pressure values and waveforms may also be reversed. This could create problems for physicians who rely on the devices to determine hemodynamic pressure and cardiac output when performing open heart surgery and other complex procedures.
If a surgeon thought the erroneous values were correct and acted accordingly, they may expose the patient to unnecessary treatments. Similarly, the lack of accurate data to inform the placement of the catheter raises the risk the surgeon will accidentally perforate a blood vessel.
FDA has received 23 reports about the device models covered by the recall notice since December 2017. Some of the reports describe problems similar to those outlined in the recall notice. In one case, a catheter provided a cardiac output value double the expected range. The device did not show an error message but the physician thought the value was suspect and replaced the catheter.
The database of reports maintained by FDA also features submissions related to inaccurate cardiac output figures and pulmonary artery pressure readings provided by devices with model numbers that are not included in the recall.
In one of these cases, the first two catheters used by the physician delivered inaccurate data but overall Edwards thinks the problem is fairly rare. Edwards produces around 1 million of the devices a year, the company told The Press-Enterprise, and a fraction of these were affected. The recall covers 1,426 devices in the U.S. from 11 lots. According to FDA's recall notice, 277 units are in commerce.
Edwards is also recalling the devices, which it makes in Puerto Rico, from other markets. Healthcare facilities in all affected regions were advised by Edwards in December to exchange devices in the recalled lots for replacement products that are free from the fault.