Dive Brief:
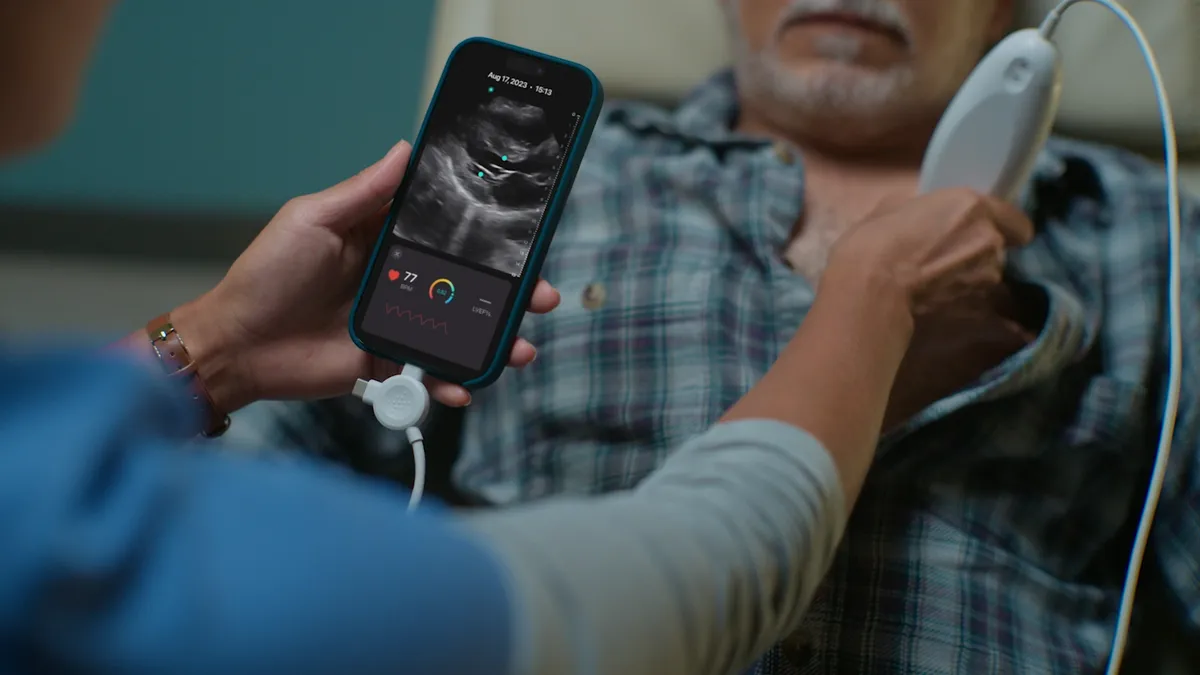
- Exo has made new artificial intelligence tools available on its Iris handheld ultrasound system, the company said Tuesday.
- The Food and Drug Administration cleared Exo’s AI tools for analyzing ultrasound images of the heart and lung last year.
- Exo sees the new capabilities as particularly beneficial for health systems and caregivers in rural and under-resourced settings because they simplify the collection and interpretation of images.
Dive Insight:
Exo received a 510(k) nod for its original Iris device in 2021 and added imaging modes and indications to the clearance the following year. The clearances cover handheld portable diagnostic ultrasound systems, similar to Butterfly Network’s iQ, that enable healthcare professionals to measure body structures and fluids in adults and children. Users can view the images on smartphone screens.
In 2023, Exo gained additional FDA clearances for AI products. One clearance covered software that uses machine learning to help quantify bladder volume from ultrasound images. Then, Exo received clearance for a broader AI platform that processes ultrasound images “to detect, measure and calculate relevant medical parameters of structures and function of adult patients with suspected disease.”
While the cleared indication is broad, Exo was more specific about the applications of the platform in its submission to the FDA. The company said the platform “provides users with a specific tool set for viewing ultrasound images of the lung and heart, placing landmarks and creating reports.”
Healthcare professionals can use an AI-assisted tool to quantify ejection fraction, a measure of blood pumped with each heartbeat, using cardiac ultrasound images. Another AI-based tool suggests the presence of “lung structures and artifacts” on ultrasound images. Trained users could perform the tasks manually and should not make patient management decisions based solely on AI analyses.
The cardiac and lung tools join applications focused on the bladder, hip and thyroid. Exo plans to double its number of FDA clearances by 2025 as the company continues to add capabilities to its Iris handheld ultrasound system.