Dive Brief:
- The Environmental Protection Agency has included facilities run by Becton Dickinson, Edwards Lifesciences and Medtronic on a list of sterilization sites that pose risks to nearby communities.
- The EPA found 23 commercial sterilization facilities that emit high levels of ethylene oxide (EtO), long-term exposure to which can increase the lifetime risk of getting cancer. Air pollution regulation intended to address EtO emissions is planned for later this year.
- Medtech trade group AdvaMed responded to the publication of the list, cautioning that EtO is vital for the prevention of serious infections and that the shutdown of facilities could disrupt the supply of medical devices.
Dive Insight:
The publication of the list is the culmination of years of focus on EtO emissions that has seen plants shut down over their environmental impact. Companies including Boston Scientific and Sterigenics began tracking their emissions under new EPA rules at the start of the year.
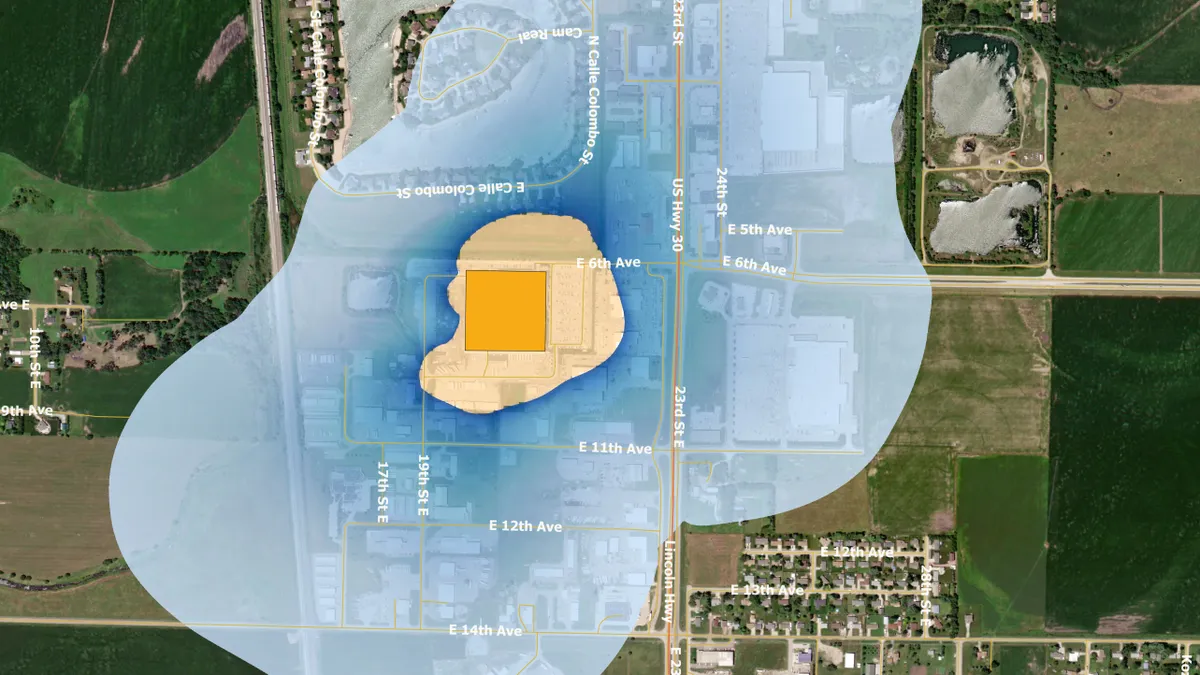
Now, the EPA has used updated EtO emissions information from commercial sterilizers to estimate the increased risk of cancer. The analysis found elevated risks at or above 100 in a million in residential areas at 23 sterilizers.
Four of the facilities, including those run by Edwards and Medtronic, are in Puerto Rico. The EPA mapped areas around the facilities in which there is an estimated lifetime cancer risk of 100 in a million or greater from breathing air containing EtO emitted from the plants. In small areas close to the facilities, the risks are higher, with the EPA putting the risk for some people near to the Edwards site at 5,000 in a million.
Two of the facilities in the continental U.S., one in Nebraska and one in Utah, are run by BD. The other facilities are run by companies including International Sterilization Laboratory, Professional Contract Sterilization and Terumo BCT Sterilization Service.
The EPA has plans to propose air pollution regulation later this year to address EtO emissions at commercial sterilizers. Ahead of the release of the regulation, AdvaMed has warned of the potential for actions against EtO emissions to cause unintended consequences that harm patients.
“The shutdown of medical device sterilization facilities due to misinformed political pressure, as well as the uncertainty regarding which regulations the facilities must adhere to as EPA works to update the federal rule, would be disastrous to public health under the best of circumstances; it could be catastrophic, in light of the fragile global supply chain, which hospitals are already strained to address,” AdvaMed CEO Scott Whitaker said in a statement.
“Our facilities are in full compliance with the Clean Air Act and all applicable laws that govern EtO sterilization,” BD spokesman Troy Kirkpatrick wrote in an email. He said the levels of ethylene oxide around BD facilities are similar to levels found in ambient air at EPA monitoring locations across the U.S., “even where there are no sterilization facilities present.” He said the firm is installing new capture and control systems at all of its EtO sterilization facilities nationwide. Once they are completed, within 12 months, he added, “All of BD’s facilities will be below the EPA’s new risk threshold for EtO emissions.”
The Food and Drug Administration, which is investigating alternative sterilization methods, responded to the EPA list, stating it “shares concerns about the release of ethylene oxide at unsafe levels into the environment” but “other methods of sterilization cannot currently replace the use of EtO for many devices.” The FDA is equally concerned about potential shortages of medical devices.
This article was updated to include a statement from Becton Dickinson.