Dive Brief:
- Elekta CEO Gustaf Salford will leave the company after the board of directors decided the manufacturer of radiotherapy machines needs new leadership.
- Salford and the board of directors have decided the CEO will leave the company on March 6, Elekta said Wednesday. The Stockholm-based firm has begun an international search for a new leader.
- Elekta disclosed Salford’s departure days after reporting its fiscal third-quarter sales, which the company called “weak” in the earnings release. Revenue fell short of expectations because of lower installations in the U.S. and challenges in China.
Dive Insight:
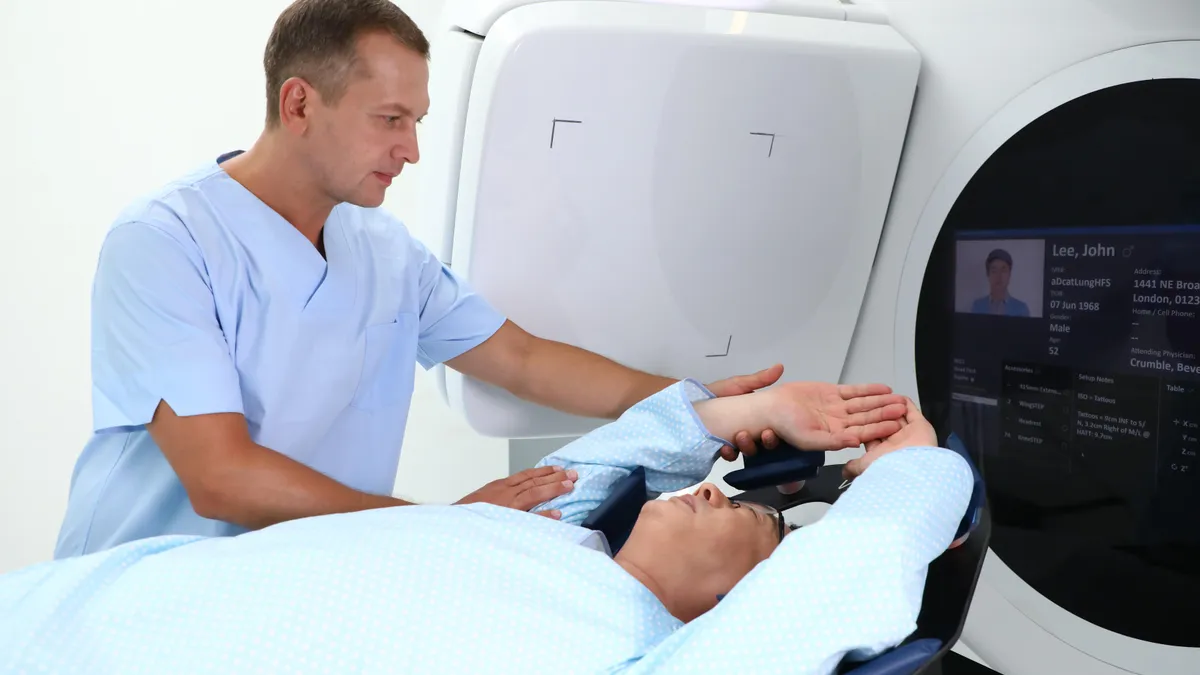
Elekta competes with Siemens Healthineers’ Varian for the radiotherapy market. Both companies make machines that combine imaging and radiation therapy to enable the targeted destruction of cancer cells.
Salford became acting CEO of Elekta in June 2020 and secured a permanent appointment five months later. Laurent Leksell, Elekta’s chairman of the board, noted the failure to meet financial expectations in a statement about the decision to look for a new CEO.
“The board of directors is of the opinion ... that Elekta now needs a new leadership that can further intensify the focus on improving profitability and strengthen growth, by leveraging the most competitive and advanced offering in the industry,” Leksell said. “This will be fueled by future product and software launches enabled by recent years’ substantial investments in R&D.”
Elekta expected sales and profitability to pick up in the second half of its financial year. However, net sales growth in the third quarter was restricted to 2% as progress in Europe and parts of the Asia Pacific region was offset by problems in the U.S. and China.
In an earnings call last week, Salford said Elekta saw “slower installation volumes” in the U.S. in the third quarter and predicted activity will remain low in the fourth quarter. Sales in the U.S. fell in the third quarter as customers waited for the regulatory clearance of the new Elekta Evo device, Salford said, and it is unclear when the Food and Drug Administration will greenlight the launch.
“We are in the FDA process right now. We get questions, we answer questions, but we cannot really say a specific date when we get clearance,” Salford said. “I think that's the main reason why you see lower installation volumes in [the] U.S. in Q3, as well as in Q4.”
In China, Salford reported “strong order growth compared to last year's low level” and “increasing public procurement activity.” However, sales in the country fell 8% in the quarter, and Salford said to expect lower volumes in China and the U.S. in the near term “as the uncertainty in the world increases.”