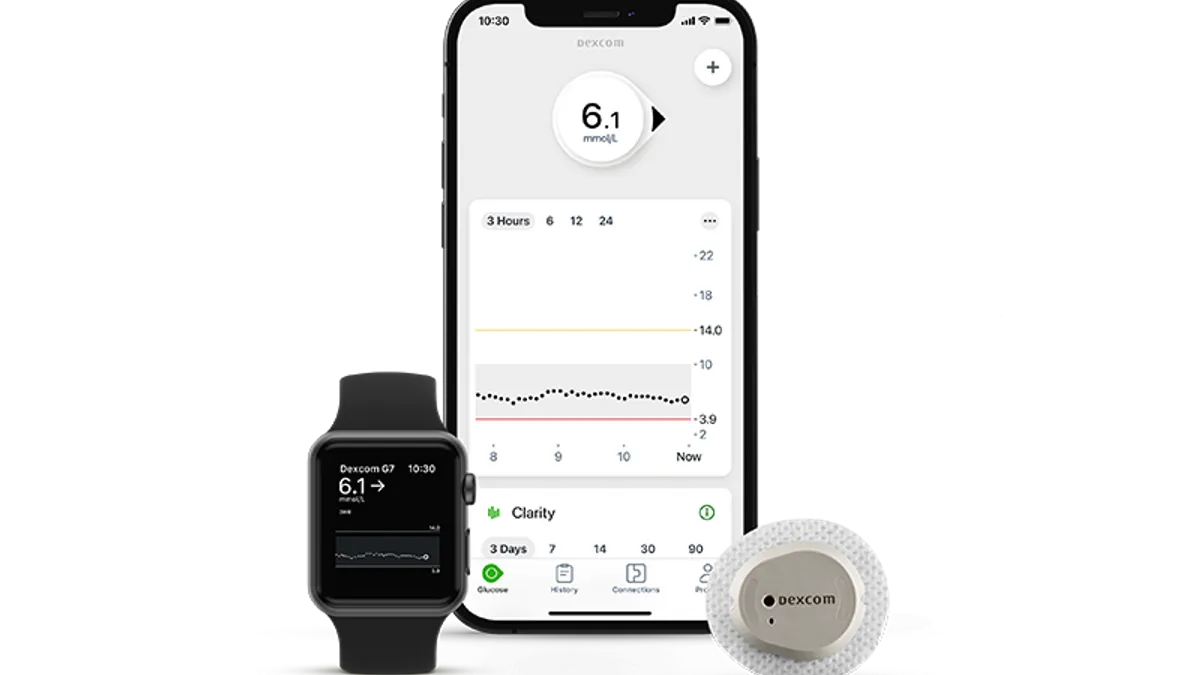
Dive Brief:
- Dexcom has begun the global rollout of its G7 continuous glucose monitoring system by making the device available in the U.K., Ireland, Germany, Austria and Hong Kong.
- The device is designed to be smaller, easier to use and have a better mobile application than its predecessor, while also powering up faster than rival CGM systems. Still the U.S. launch has been delayed, until late 2022.
- Dexcom received a European CE mark for G7 in March, boosting its attempt to win market share from chief rival Abbott, which is stepping up the European rollout of its latest FreeStyle Libre 3 CGM device.
Dive Insight:
Dexcom is pushing the startup time as a key differentiator over Abbott’s FreeStyle Libre. While G6 took two hours to warm up, putting it at a disadvantage versus Abbott’s Libre sensor, which takes one hour to acclimatize the body before it starts delivering glucose readings, Dexcom now has an edge over its rival.
“G7 offers a 30-minute warmup time, faster than any other glucose sensor on the market. This gives you back 90 minutes of glucose data every time you change a sensor. That's time you can spend doing what you love instead of worrying as much about your numbers. With the push of a button, you'll see glucose on your phone in 30 minutes,” Jake Leach, Dexcom’s chief operating officer, said at the launch event.
The G7 sensor is now available for use by people with diabetes aged two years and older in the U.K., Ireland, Germany, Austria and Hong Kong. Dexcom is working to launch the device in New Zealand and South Africa in the weeks ahead with more markets to follow.
It will take Dexcom longer to bring the G7 to patients in its biggest market. The U.S. accounted for 73% of Dexcom sales in the first half of the year even as the need to update G7 software has delayed the launch of the latest CGM in the country. Dexcom is aiming to win approval from the Food and Drug Administration this year, enabling a limited U.S. rollout in 2022 ahead of a full launch next year.