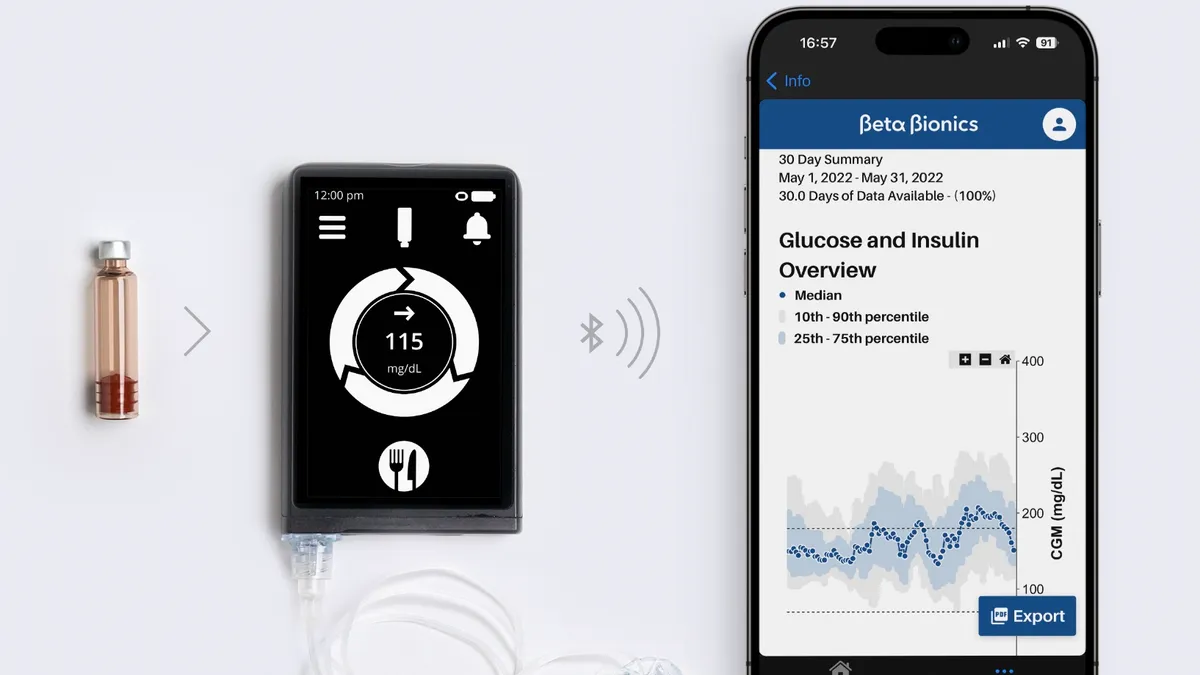
Dexcom has led the latest batch of FDA breakthrough device designations, securing the regulatory privileges for a version of its continuous glucose monitor technology designed for use in hospital settings.
After the start of the COVID-19 pandemic, the FDA issued guidance allowing glucose monitors indicated for home use to be used in a hospital setting, letting patients track and report their own blood glucose levels to reduce health care workers' exposure to the virus. Now, Dexcom is seeking clearance for its CGMs to be used in a hospital setting.
The breakthrough designation covers a small, wearable sensor that continuously measures glucose levels and a linked transmitter that sends the data wirelessly to a smart device. In doing so, the system provides real-time glucose data without needing to take samples using finger sticks. Users can set customizable alerts for when a patient has dangerously high or low blood glucose levels.
Those features have enabled Dexcom to capture a slice of the outpatient market for CGMs. In recent years, Dexcom and its partners have laid the groundwork for the expansion of the use of the device into hospital settings, notably when FDA relaxed the rules on its use by inpatients during the pandemic.
"In our extensive use of Dexcom CGM in our hospitals as part of exploratory studies over the last seven years, more than 800 of those patients treated during the pandemic, we have found that the device improves glucose control without any increased risk in hypoglycemia," Athena Philis-Tsimikas, an endocrinologist and corporate vice president for the Scripps Whittier Diabetes Institute in San Diego, said in a statement.
Dexcom is one of a handful of companies to disclose breakthrough device designations so far this month. Insightec received the status for its Exablate Neuro system in the treatment of non-small cell lung cancer (NSCLC). The device uses noninvasive, low-intensity focused ultrasound to try to open up the blood-brain barrier, thereby enabling treatments to reach the sites of hard-to-treat NSCLC metastases.
Insightec disclosed the breakthrough designation alongside news that FDA has granted its request to trial the device in combination with Merck's checkpoint inhibitor Keytruda in NSCLC that has metastasized to the brain, and to enhance the efficacy of liquid biopsy for recurrence monitoring of patients with primary brain cancer. The device is already approved for use in the treatment of essential tremor and Parkinson's disease.
FDA granted breakthrough status to Merit Medical Systems' Embosphere microspheres for use in the genicular artery embolization (GAE) of patients with symptomatic knee osteoarthritis. Surgeons perform GAE to reduce blood flow to the knee and thereby reduce pain and disability caused by inflammation. Merit's microspheres are already used to occlude blood vessels for other purposes.
AltPep received breakthrough status for a blood test to detect Alzheimer's disease. The SOBA-AD assay is designed to detect toxic forms of the amyloid-beta peptide that aggregates in patients with the disease at an early stage in the progression of Alzheimer's. AltPep's long-term vision is to detect the disease even before symptoms occur and thereby potentially enable treatments that limit cognitive decline.
CardioStory secured the FDA designation for a non-invasive filling pressure measurement and monitoring platform for use in heart failure patients. The breakthrough status follows the completion of a study that found the noninvasive option had 89% accuracy compared to the invasive, gold-standard approach to the measurement of pulmonary capillary wedge pressure.
Finally, Georgia Institute of Technology hailed the contribution of its researcher Omer Inan and Emory University psychiatrist Douglas Bremner to a breakthrough designation for electroCore's non-invasive vagus nerve stimulation device to reduce PTSD symptoms. The company disclosed the breakthrough status in January.