Dive Brief:
-
CMS on Wednesday laid out proposed coverage changes for ventricular assist devices (VADs) and artificial hearts, responding to formal requests from device makers Abbott and SynCardia in 2019 to take a fresh look at reimbursement policies for the advanced heart failure technologies.
-
If the proposal is finalized, CMS will remove the current “coverage with evidence development” requirement on artificial hearts and leave coverage determinations up to local Medicare Administrative Contractors. As for VADs, CMS sided with Abbott's urging to move coverage basis away from strict "bridge to transplant" or "destination therapy" classifications. CMS is "proposing to modify the [national coverage determination] to update the patient selection criteria irrespective of therapeutic intent category."
-
The agency will accept public comments until Sept. 12 and CMS is set to issue a final NCD by Nov. 10. Analysts at Cowen Washington Research Group “don’t expect any significant changes from the proposal,” according to a note Wednesday.
Dive Insight:
The proposals come years since the agency last revisited its policies on artificial hearts in 2008 and on ventricular assist devices in 2013. Both categories of mechanical circulatory support devices are meant to help patients with end-stage heart failure.
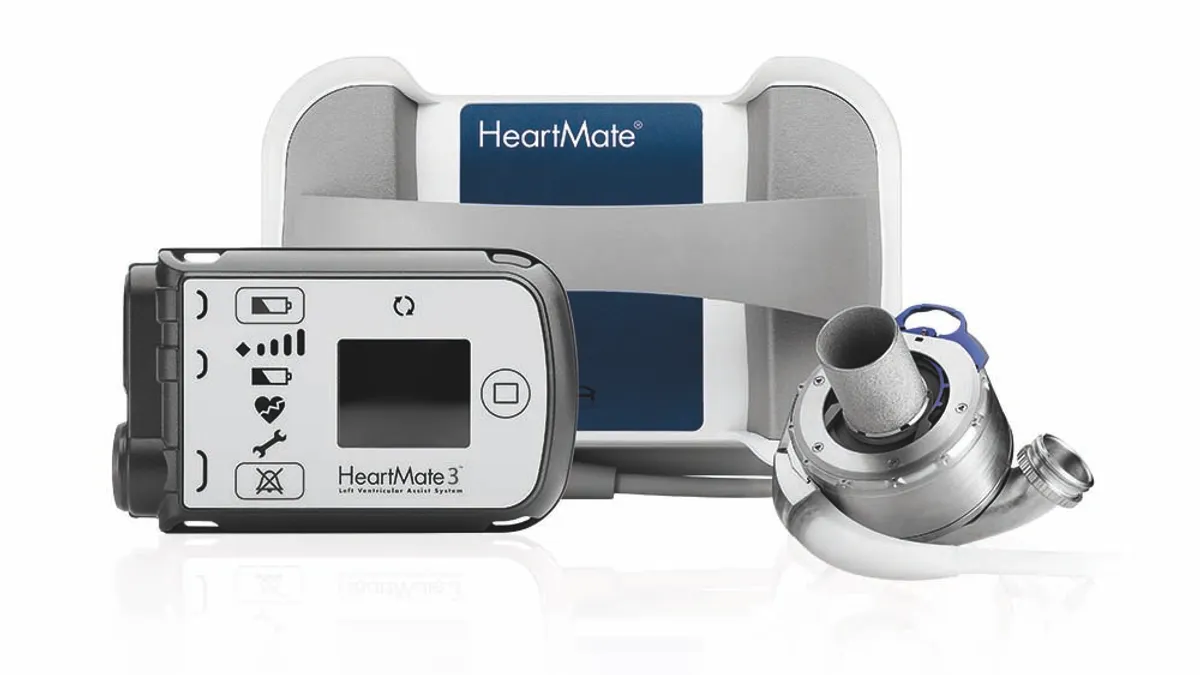
While privately-held SynCardia still can claim that it's the only medtech with an FDA-approved total artificial heart, there have been developments in the VAD market. Abbott launched the third-generation of the HeartMate LVAD system in 2017, having gained an earlier version of the technology through the acquisition of St. Jude Medical. Medtronic and Berlin Heart have their own offerings.
During the February to March comment period earlier this year, the changes drew responses from each of those companies. Also weighing in with mixed feedback were AdvaMed, the American Heart Association, Society of Thoracic Surgeons, American College of Cardiology and heart failure experts from health systems across the country.
Regarding artificial hearts, CMS Administrator Seema Verma said the updated criteria "better reflects" the needs of some 6.5 million U.S. adults living with heart failure.
In deciding to lift the evidence development requirement, CMS said in its draft that research on artificial hearts had shown improved survival for patients with severe biventricular failure, but that due to the very low quantity of patients who receive such devices (less than 1% of the Medicare population), local determinations are more suitable than a national coverage policy.
“MACs are structured to take into account a beneficiary’s particular clinical circumstances to determine which patients will benefit from receiving an artificial heart,” CMS noted in a press release. SynCardia did not have comment in time for publication.
Notably, different cardiology groups had diverged during the spring comment period on whether CMS should keep the coverage with evidence development stipulation, approval for use only in the context of a clinical trial.
The American Heart Association backs lifting that caveat, while the Society for Thoracic Surgeons, American College of Cardiology, Heart Failure Society of America and American Association for Thoracic Surgery argued the opposite, citing many outstanding clinical questions on artificial hearts and a need for further data collection.
As for the potential changes on ventricular assist devices, CMS agreed that Abbott's MOMENTUM 3 trial, which supported FDA approval of HeartMate 3 in 2018, effectively questioned "the clinical relevance of characterizing patients pre-implant," noting that patients "often transition between device strategies following device implant."
CMS wants to better meet that need for flexibility. "We believe these proposed changes will facilitate better access and clinical care regardless of whether patients start in one therapeutic intent category or another, or dynamically move between them," the agency said in its draft.
A Medtronic spokesperson said in an email the company "is pleased that CMS’ proposed national coverage decision memorandum for VADs will preserve patient access for all labeled LVADs currently on the market."
Likewise, Abbott's Robert Kormos, divisional vice president of global medical affairs for heart failure, called CMS' proposal regarding VADs "very promising for people living with advanced heart failure."