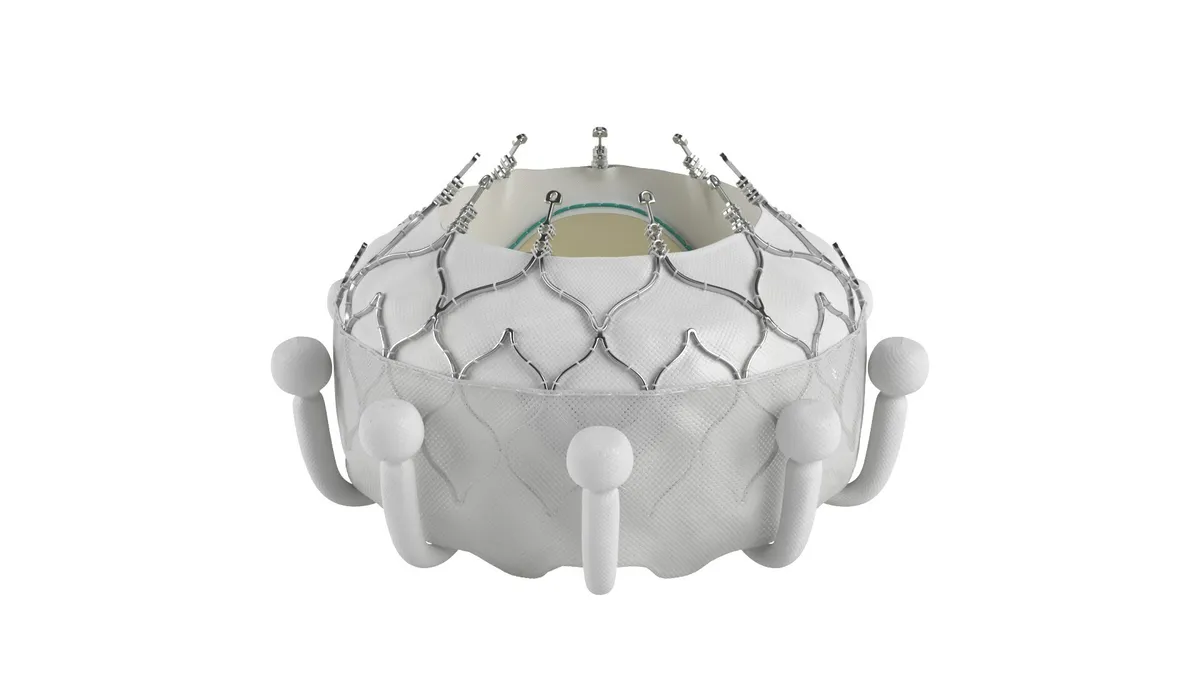
The Centers for Medicare and Medicaid Services issued a proposed decision memo on Thursday for Medicare to cover transcatheter tricuspid valve replacement devices. The decision could benefit Edwards Lifesciences, which is seeking a national coverage determination for its Evoque heart valve.
The CMS proposed coverage for patients with severe, symptomatic tricuspid regurgitation, a condition where the tricuspid valve doesn’t close properly, allowing blood to leak back into the heart’s left atrium. The agency also plans to use its Coverage with Evidence Development program, which allows for Medicare coverage of a treatment on the condition that data is gathered through a clinical trial or registry to determine its effectiveness.
The CMS proposed that studies should consider all-cause mortality or hospitalizations through a minimum of 24 months.
Edwards’ Evoque device is an artificial valve made of cow tissue attached to a self-expanding metal frame. The procedure is conducted using a catheter, intended as an alternative to open-heart surgery. Edwards received Food and Drug Administration approval for the device in February and requested a national coverage determination later that month.
Edwards said the proposed national coverage determination provides a pathway for access to the technology and that it will submit a public comment, company spokesperson Loree Bowen wrote in an emailed statement on Friday.
Daveen Chopra, leader of Edwards’ transcatheter mitral and tricuspid therapies unit, told investors in October that the company hopes to see a draft national coverage determination for Evoque by the end of year and a final decision in the first quarter.
Evoque became eligible for Medicare’s new technology add-on payment (NTAP) in October, allowing certain hospital centers to receive payments above standard reimbursement, CEO Bernard Zovighian told investors. The NTAP would be in effect for three years.
Chopra added that the national coverage determination “will help determine which patients and which centers do Evoque, and which patients would get reimbursed. The NTAP helps increase the amount of payment for each patient when they do a case.”
In comments submitted to the CMS during the summer, some physicians and consumer advocacy group Public Citizen called for the agency to convene a Medicare Evidence Development & Coverage Advisory Committee meeting before issuing a coverage decision. They raised concerns about the unblinded nature of Edwards’ pivotal Triscend II study, as well as the fact that the treatment group had a 27.4% rate of major adverse events after 30 days.
Other physicians, who participated in studies of the device, called for coverage of Evoque. An interventional cardiologist at Piedmont Heart Institute wrote, “Our patients have done dramatically well.”
The CMS is seeking public comment on the proposed decision memo through Jan. 18, 2025.
Editor’s note: This article has been updated with comments from Edwards.