Dive Brief:
-
CMS has finalized Medicare policies for artificial hearts and ventricular assist devices (VADs) in an update expected to expand coverage.
-
The final policy released late Tuesday, which stemmed from requests by Abbott and SynCardia, hews closely to the draft CMS released for consultation in August. CMS has tweaked the wording of the patient selection criteria but otherwise largely retained the proposed text.
-
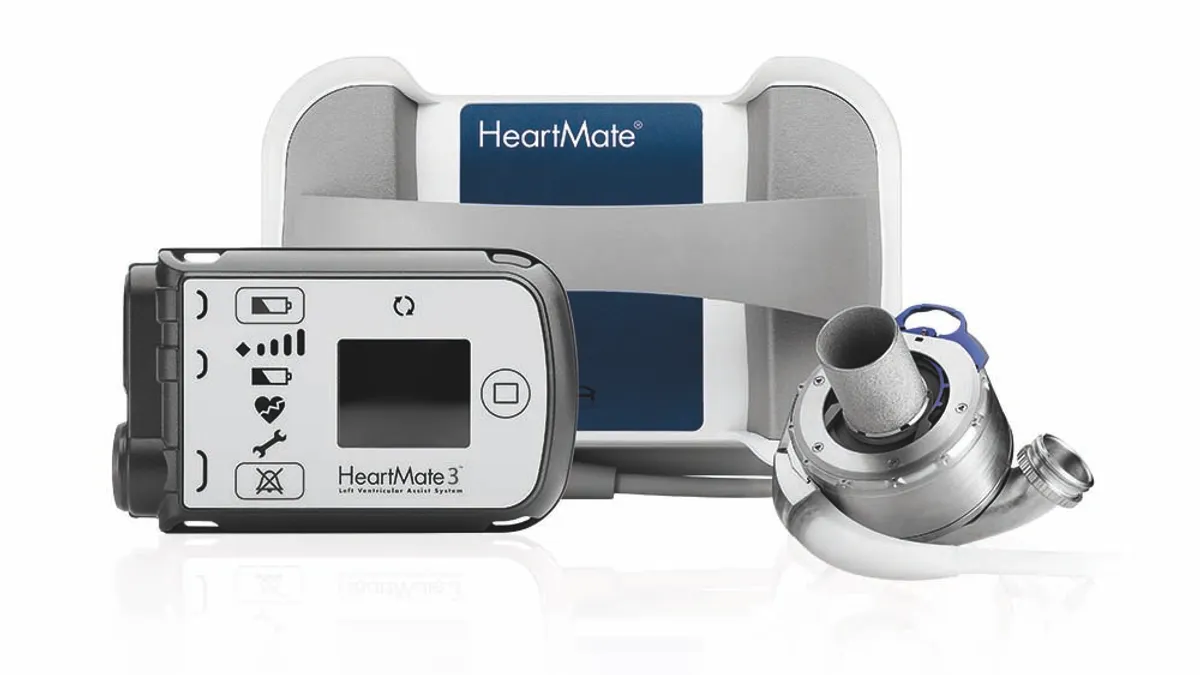
Under the new policy, Medicare will cover artificial hearts outside of clinical studies and align its VAD coverage criteria with current medical practice, positioning more people to use products such as Abbott's HeartMate 3. Cowen analysts in a Wednesday note said the CMS decision will "apply equally" to Medtronic's LVAD system, theHeartWare HVAD, and they see it as "favorable" to both companies.
Dive Insight:
The old VAD national coverage determination (NCD) split the patient population up into people who received the device as a bridge-to-transplant (BTT) and others who got the implant as a destination therapy. Cardiologists were scathing of the approach when consulted by CMS earlier this year, calling it "artificial," "antiquated" and "archaic."
CMS responded to the feedback with an August draft that proposed removing the BTT requirements. The final text released Tuesday is largely unchanged from the August draft. CMS has simplified the patient selection criteria by dropping a prescriptive list of requirements in favor of the statement that the criteria "reflect the major inclusion criteria of contemporary trials." The longer, more prescriptive statement is still included elsewhere in the text, though.
The sections of the policy on artificial hearts are unchanged, too. That means CMS is ending the need for coverage with evidence development for artificial hearts. The action clears Medicare Administrative Contractors to make coverage determinations for the devices.
CMS largely stuck with the proposed text despite receiving 42 comments on the drafts. Dissenting voices raised concerns that removing the NCD for artificial hearts may limit or delay access to procedures and create inconsistent coverage. CMS said it understands the concerns regarding access but thinks its policy supports appropriate coverage. Based on current evidence, CMS said it cannot establish clear criteria for national coverage of artificial hearts.
The evidence on VADs is stronger, in CMS' view, leading the agency to provide patient selection criteria. CMS received some pushback against the criteria during the latest comment period, with multiple respondents proposing alternatives. In response, CMS said it is retaining the original criteria as they should lead to the use of VADs in patients with similar attributes to participants in successful clinical trials such as Abbott's MOMENTUM 3.
Other respondents spoke out against CMS’ proposal to end the need for implanting sites to receive written permission from a Medicare-approved transplant center before using a VAD. The critics argued the change could lead patients eligible for heart transplants to receive a VAD instead. CMS said physicians should consult with heart transplant specialists but that the details of the process are beyond the scope of the NCD.
Cowen analysts believe the coverage changes will "give surgeons additional flexibility in their use of LVADs by defining the need for support in less rigid terms."
The changes could benefit Abbott and SynCardia, the companies that triggered the review of the old policies, and companies with similar devices such as Medtronic. Ultimately, CMS said the VAD changes "will expand coverage to a greater number of candidates who are likely to benefit from this technology."