Dive Brief:
- Cardinal Health marketed convenience kits that include piston syringes not authorized by the Food and Drug Administration, the agency said in an April 24 warning letter.
- The agency found the violations during an inspection of Cardinal’s facility in Waukegan, Illinois. Cardinal distributed syringes made by Jiangsu Shenli Medical Production with “substantially different technological characteristics” than those cleared by the FDA, according to the letter.
- Cardinal has come under scrutiny after the company recalled some of its disposable syringes last year because their dimensions had changed, making them incompatible with certain infusion pumps. The FDA has also taken steps to block plastic syringes made by Jiangsu Shenli from being imported.
Dive Insight:
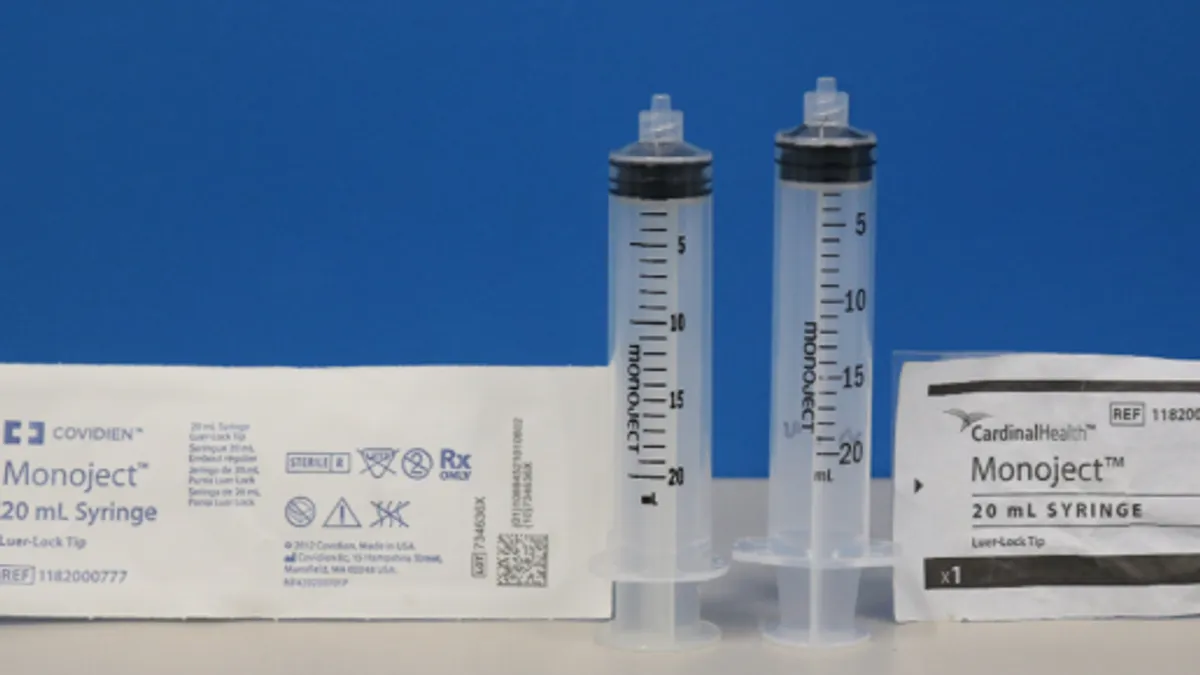
The warning letter focuses on Cardinal’s Monoject luer-lock tip syringes, which are intended to inject fluids into the body or withdraw fluids, and Monoject enteral feeding syringes, which deliver fluid, food or medications to a patient’s feeding tube.
Cardinal has 510(k) clearance for a 5 mL luer-lock piston syringe made by Jiangsu Shenli, but it also sold convenience kits with 3 mL, 10 mL and 20 mL piston syringes that had not been cleared by the FDA.
In addition to the different sizes, the kits included control syringes, which the FDA said constitutes a significant design change and could affect the safety or effectiveness of the finished product.
“Specifically, changing the volume of the syringe and changing from a traditional syringe to a control syringe could result in the risk of patient harm such as inaccurate dosing and a leaking device,” the agency wrote in the warning letter.
Cardinal also failed to ensure its products conformed to good manufacturing practices, the FDA said. For example, the company was not able to produce data to support the use of the syringes with infusion pumps.
Additionally, the FDA found during the inspection that Cardinal had self-manufactured luer-lock syringes of various sizes based on specifications cleared by the agency. Cardinal then switched to overseas manufacturing with Jiangsu Caina Medical, a separate firm from Jiangsu Shenli, to produce the same range of syringes.
“Since switching to overseas manufacturing there have been increases in reported syringe safety issues regarding syringe/infusion pump compatibility resulting in your firm’s ongoing syringe recalls,” the agency wrote in the warning letter.
Jiangsu Caina and Jiangsu Shenli are manufacturers the FDA has scrutinized as it probes plastic syringes made in China. The agency recently warned medical device companies to immediately transition away from using plastic syringes made by the firms. The FDA also issued import alerts, which allow it to detain products.
Cardinal has stopped marketing the syringes as compatible with infusion pumps and has taken several actions to revise and review its internal policies for device marketing, according to the warning letter. But those actions have not yet been evaluated for effectiveness, so the FDA will need to do a follow-up inspection.
The agency also asked Cardinal to detail in writing what actions it has taken and plans to take regarding the use of syringes with infusion pumps.