Dive Brief:
- Boston Scientific on Thursday received approval from the Food and Drug Administration for a new heart ablation device to treat atrial fibrillation, according to a company announcement.
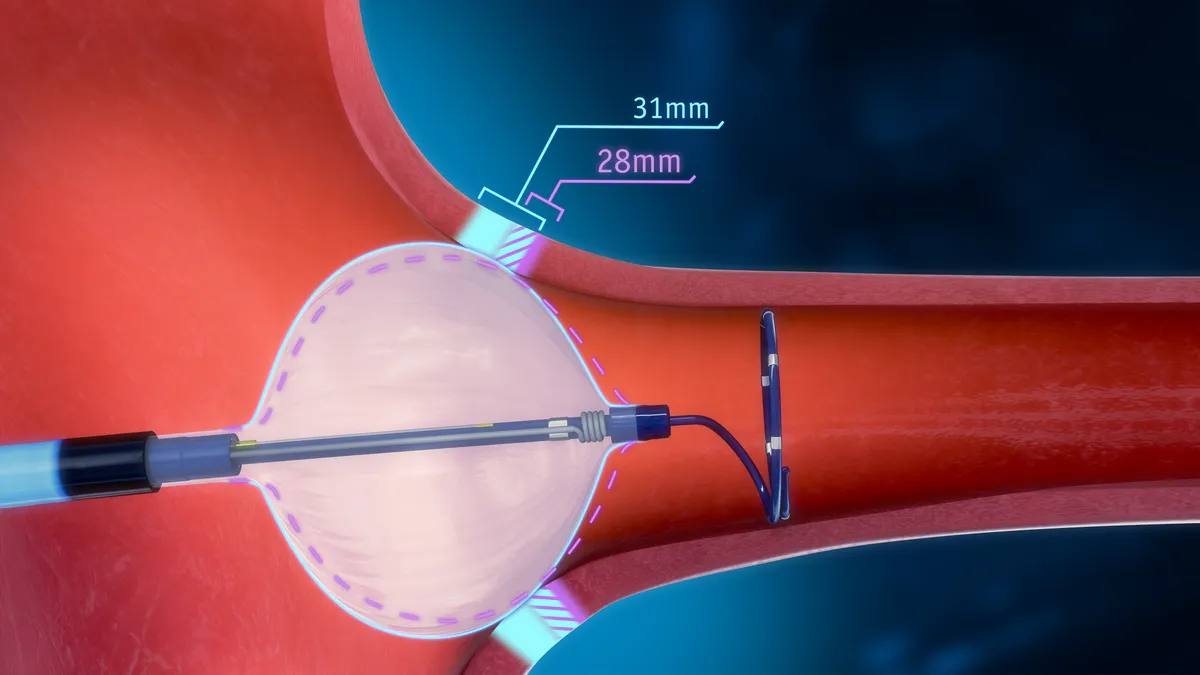
- The POLARx Cryoablation System can accommodate two balloon sizes in one catheter, which Boston Scientific said allows physicians to tailor care to individual patients.
- The device has been selling well in Europe and Japan, where it is cleared for use, CEO Mike Mahoney said in an earnings call last month. Boston Scientific has been competing with companies like Johnson & Johnson and Medtronic, which are also bringing new cardiac ablation devices to market.
Dive Insight:
Cryoablation is a minimally invasive procedure that uses a balloon catheter to freeze tissue near the pulmonary vein. Scars then block irregular electric signals that can cause atrial fibrillation.
Switching between two balloon sizes, clinicians can adjust the device to a patient’s anatomy during a procedure, mitigating device change-outs, Boston Scientific said in a statement. The device can also let them treat a wider range of pulmonary vein anatomies.
The POLARx system received approval in Europe in 2020 and in Japan in 2021. To date, it has been used in more than 25,000 patients worldwide, Boston Scientific Electrophysiology President Nick Spadea-Anello said in a statement.
Boston Scientific’s electrophysiology sales grew 28% in the second quarter, driven by strong international performance from the company’s POLARx and FARAPULSE cardiac ablation devices, Mahoney said in a call with investors last month.
FARAPULSE, which uses pulsed field ablation to treat heart arrhythmias, received a CE Mark in 2021 but isn’t yet approved in the U.S. Positive safety data is expected to draw more clinicians to pulsed field ablation in the future.
“The cryo [ablation] market is quite substantial in the U.S. And certainly, doctors are very interested in FARAPULSE, but there's a number of cryo users there,” Mahoney said on the earnings call. “Some physicians may be looking for even longer-term data on FARAPULSE. So we do see a nice market for cryo in the coming years.”
Approval of POLARx was based on the results of a prospective, single-arm study which found that 96% of patients did not experience a safety event such as pulmonary vein stenosis or esophageal fistulas after a year. Of the 385 people who participated in the trial, 80% were free from atrial arrhythmias after a year.
Meanwhile, competitors are running their own cardiac ablation studies and bringing new devices to market.
J&J recently received an expanded indication for some of its radiofrequency ablation devices to be used without fluoroscopy imaging, reducing patients’ radiation exposure. And in March, Medtronic received a CE Mark for its Affera device that allows physicians to choose between pulsed field ablation and radiofrequency ablation.