Dive Brief:
- German medical device company Biotronik said Friday it received premarket approval from FDA for its Orsiro ultrathin drug-eluting stent intended for use in percutaneous coronary intervention procedures.
- The PMA application was backed by data from an international randomized trial with two-year follow up funded by Biotronik, which put Orsiro head-to-head with clinical standard Xience stents from Abbott and showed lower rates of target lesion failure and target vessel myocardial infarction, or heart attack, after 12 months among patients who received the Biotronik device.
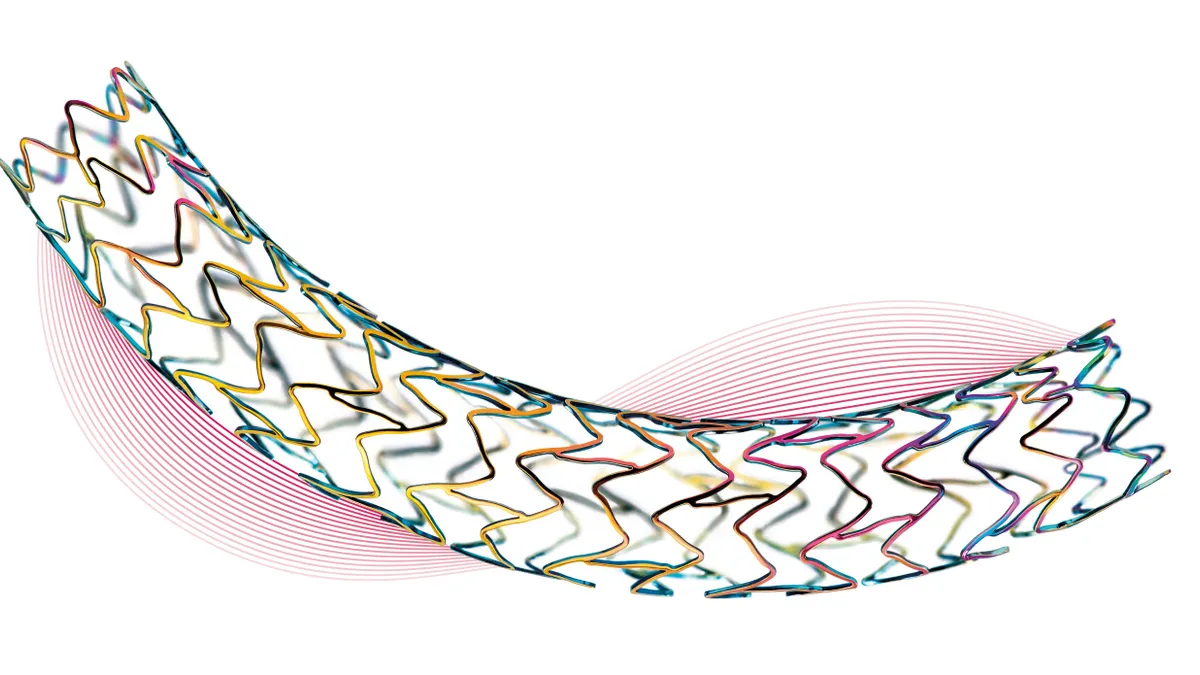
- The cardiac rhythm management, electrophysiology and vascular intervention company's design incorporates an ultrathin (strut of 60 μm) cobalt chromium metal stent with a bioresorbable polymer coating that elutes immunosuppressive drug sirolimus (BP SES).
Dive Insight:
These drug-eluting stents are supportive metal mesh tubes inserted into the coronary artery that offer timed release of medication to prevent against the artery becoming blocked again.
FDA said in its approval letter the Biotronik device, which has been CE-marked since 2011, is specifically indicated for improving coronary luminal diameter, including in patients with diabetes mellitus, with symptomatic heart disease, stable or unstable angina, nonST elevation myocardial infarction or documented silent ischemia due to atherosclerotic lesions in the native coronary arteries, with a reference vessel diameter of 2.25 mm to 4.0 mm and a lesion length of ≤ 36 mm.
The BIOFLOW-V study compared Biotronik's design with Abbott's Xience system, which is a durable polymer everolimus-eluting stent (DP EES) with a thin strut of 81 μm. The first generation of Xience was approved in 2007 after review by FDA's Circulatory System Devices Advisory Panel.
1,334 patients received randomized treatment, with 884 receiving the BP SES and demonstrating a 7.5% target lesion failure rate after two years, compared to the 450 who received the DP EES and showed a rate of 11.9%. Among other heart health metrics, rates of cardiac death or myocardial infarction were 7.0% in the BP SES group, versus 10.4% in the DP EES group. Late or very late definite stent thrombosis was 0.1% for Orsiro users and 1.0% for Xience patients.
Medtronic conducted a similar head-to-head study with Orsiro, touting results last year that showed non-inferiority in its Resolute Onyx stent, which launched in 2017, compared to Biotronik's product.
With the U.S. marketing authorization hurdle cleared, Biotronik suggested it's looking to become the new standard-of-care option among drug-eluting stents, saying that the market had become "highly commoditized."
Biotronik got an FDA approval last September through the humanitarian device exemption review process for its PK Papyrus system, a repair-oriented device used in the life-threatening event that a tear in the coronary artery occurs during a procedure to clear blockages.
Biotronik said Orsiro is now commercially available in the U.S. in 52 diameter sizes and lengths up to 40 mm, which it says are the longest available in the U.S. market.