Dive Brief:
- Becton Dickinson formed a partnership to co-sell and co-promote Magnolia Medical's Steripath platform.
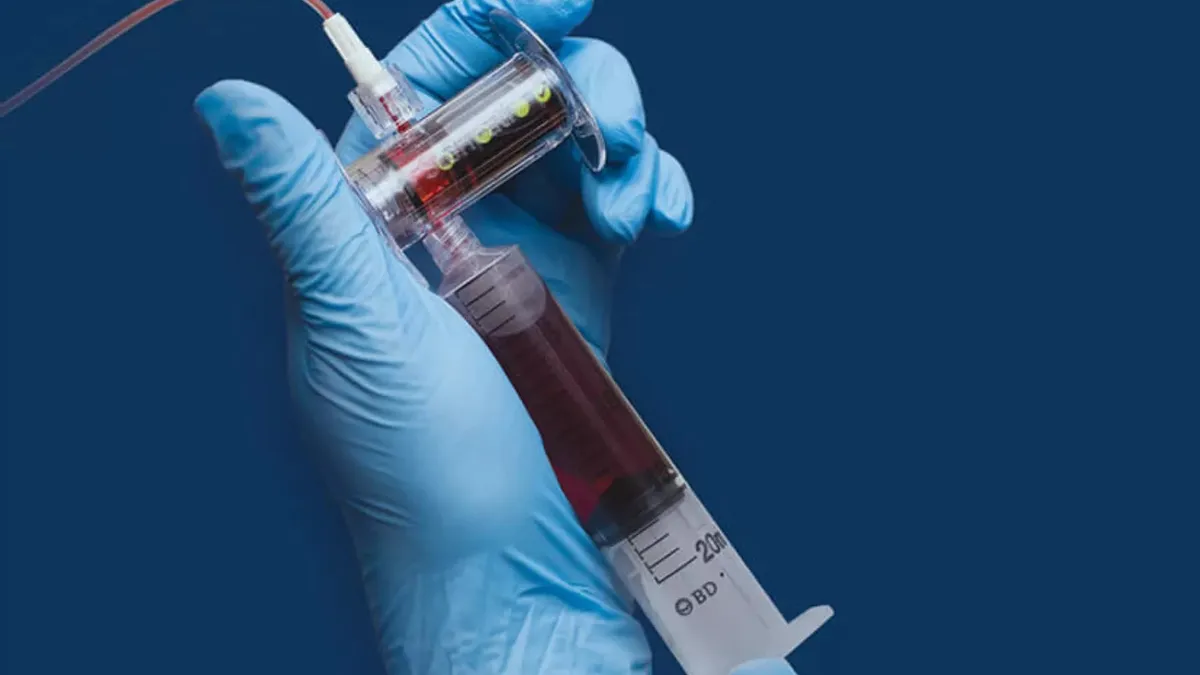
- Steripath is a 510(k)-cleared platform designed to reduce blood culture contamination and improve sepsis testing accuracy. The deal also covers Steripath Micro, a version designed for use in children and patients with difficult intravenous access, the companies said in a statement on BD’s website.
- BD sees the Magnolia devices as complementary to its own specimen collection portfolio and a way help efforts by the Centers for Disease Control and Prevention to reduce blood culture contamination.
Dive Insight:
The common failure to fully sterilize skin before drawing blood often leaves the first drops of blood contaminated by tiny flecks of skin. That can contaminate blood samples, especially when testing for sepsis. The Steripath platforms divert the initial small volume of potentially contaminated blood, 1.5 milliliters to 2 milliliters in the case of the standard device, isolating it from the rest of the blood sample.
Investigators have validated the technology in prospective clinical research and real-world use, enabling Magnolia to make the Food and Drug Administration-cleared labeling claim of an 83% and 88% reduction in contamination rates.
With CDC guidelines recommending the diversion of the initial 1.5 milliliters to 2 milliliters of blood, BD sees a chance to grow sales of the Magnolia technology. The co-exclusive commercial agreement positions BD to pair its technologies with the Steripath platforms
“By offering a combined innovative technology solution with the Steripath Initial Specimen Diversion Device platform and BD Vacutainer push-button and BD Vacutainer UltraTouch blood collection sets, we are aligning our shared commitment to improve patient outcomes, help hospitals achieve their quality goals and reduce unnecessary hospital costs,” Magnolia CEO Greg Bullington said in the statement.