A relative rarity a decade ago, robot-assisted surgery may be going mainstream. A JAMA analysis published last month suggested as many as 15% of general surgeries may now be performed with the help of a robot, rising from roughly 2% over the course of six years.
For industry juggernaut Intuitive Surgical, total procedures using the da Vinci robot surged 18% in 2019 to nearly 1.23 million surgeries worldwide. The company has enjoyed sparse commercial competition since receiving FDA clearance for da Vinci two decades ago, but across the medtech landscape, investment in similar platform technologies is heating up.
Medtronic, Johnson & Johnson, Siemens Healthineers, Stryker, Zimmer Biomet and others have systems in various stages of development or commercialization, as robot-assisted surgery moves beyond general surgery into more novel areas such as lung biopsy.
"The rising investment in the sector will continue to boost both awareness of the technology and the numbers of surgeons trained on robotic surgery platforms," EY said in a recent industry report. And in the meantime, "intensifying competition will bring down the costs associated with robotic surgery.”
Here are four of the areas where that competition is playing out.
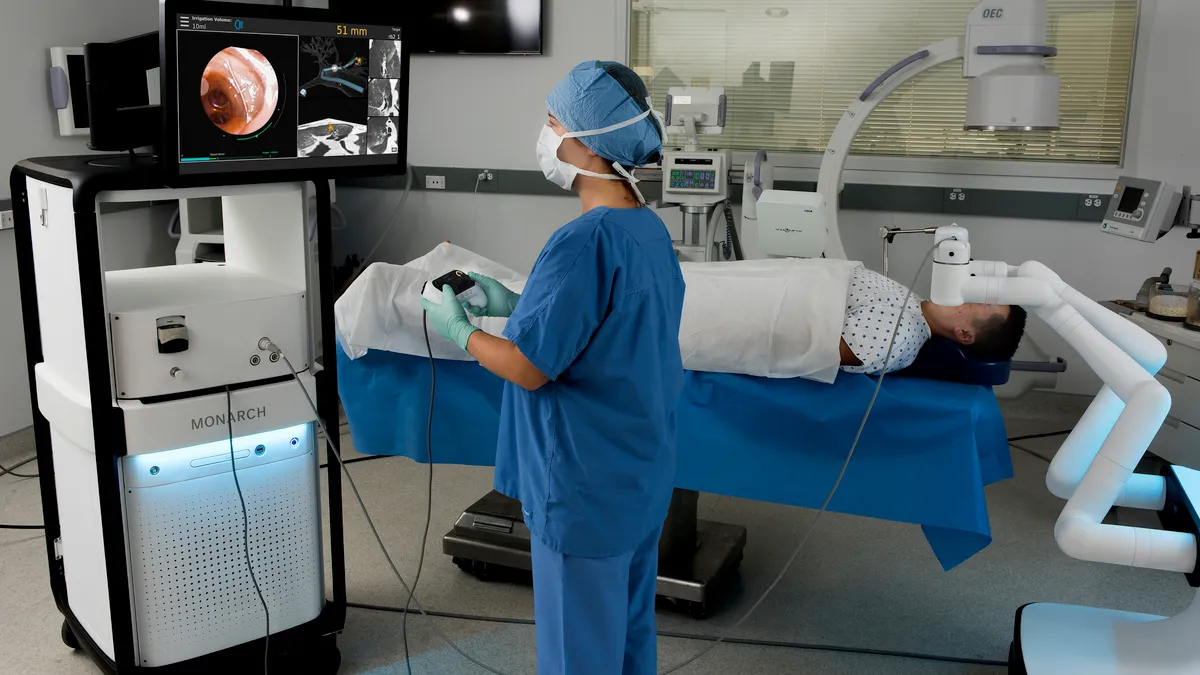
Lung biopsy attracts Intuitive, J&J
J&J’s $3.4 billion purchase of Auris Health in 2019 gave the medtech giant a robotic diagnostic and therapeutic endoscopy platform called Monarch for applications in lung cancer. As part of the deal, J&J also gained the services of former Auris CEO and founder of Intuitive Frederic Moll and his team.
J&J CEO Alex Gorsky said in January its Auris and Verb Surgical teams are collaborating to build a platform that “will be in place for the next several decades.” More details on the project are slated to be shared at the company's medical devices investor day on May 13.
Meanwhile, Intuitive is looking to bolster the case for its technologies to address a wider range of conditions, including lung cancer. The company is studying an approach to robotic surgery with its Ion platform for lung biopsy of suspicious nodules that involves access through the patient’s mouth. Separate studies are evaluating lobectomy in lung cancer patients using da Vinci, spokesman Eric Torbenson told MedTech Dive in an email.
Major medtechs take aim at da Vinci's dominance
With erosion in their traditional surgical instruments businesses, both J&J and Medtronic are investing heavily in soft tissue robotic systems to go head to head with da Vinci, and are expected to compete on price.
Medtronic CEO Omar Ishrak this month said the company’s effort to bring a soft tissue robot, dubbed Hugo, to market is on track, but did not give an update on the timeline to launch.
Following an event for analysts in September, Needham & Company’s Mike Matson wrote that Medtronic could have a limited early launch of its answer to da Vinci outside the U.S. in the second half of the company's fiscal 2020. CE mark and U.S. investigational device exemption filings are expected during the first half of fiscal 2021. The IDE exemption would support clinical trials geared for U.S. approval.
Also this month, Medtronic announced the acquisition of Digital Surgery, bolstering its robotic surgery division with a company that makes an interactive surgical simulator.
J&J, which stepped up its robotics efforts at the end of 2019 with the buyout of Verb Surgical, was also tightlipped on its launch timeline during its most recent earnings call, but executives noted the opportunity. “If you think ahead in medical devices, there are probably few things that are going to represent a more secular shift in that domain than the shift into digital and robotic surgery,” Gorsky said in January.
Still, Morningstar analyst Debbie Wang said it will be hard for Medtronic and J&J to catch up.
"Intuitive has had this huge head start, so as a result it has for the most part exhausted all the green fields, the untapped hospitals. It’s entrenched," she told MedTech Dive.
As its deep-pocketed rivals move closer to launching robotic platforms, Intuitive is moving ahead with a strategy to maintain market dominance. The company is currently in the first phase of a rollout for its da Vinci SP, or single port, platform and is pursuing additional clearances for the system, including colorectal procedures.
In addition to growth in colorectal procedures, Intuitive expects a growing number of bariatric procedures and hernia repairs, as well as expansion outside the U.S., to drive double-digit procedure growth in the year ahead, albeit at a somewhat slower pace than last year.
The introduction of its flexible diagnostics platform, Ion, is also underway, and Intuitive expects several publications evaluating the performance of that system to be presented in 2020.
The company is boosting imaging and analytics capabilities with additional investments in 2020. An augmented reality system called IRIS that simulates surgery began clinical use in the fourth quarter. Intuitive is also looking to ramp sales in China after recently gaining approval there.
The robotics pioneer is further focused on building da Vinci's body of clinical data and expects more than 1,000 studies to be published this year on use of the system, Torbenson said. Preliminary data from a clinical study on Ion is also due out, he said.
Also looking to challenge Intuitive's general surgery stronghold is U.K.-based CMR Surgical, which launched its Versius robotic system in India last fall and is in the process of applying for FDA clearance for its platform, according to a company spokeswoman. The robot is designed to assist surgeons in gynecology, general surgery, upper GI and colorectal procedures.
J&J to enter the orthopaedics fray, as Zimmer Biomet chases Stryker
Stryker jumped out in front in robotics for orthopaedic surgery with the U.S. launch in 2017 of its Mako system for total knee replacements. Now, demand for the robot is driving market share gains for the company's knee devices, executives said last month.
The fourth quarter of 2019 represented the platform's strongest performance to date, with robotic procedures, including hip and partial knee replacements, climbing 50%. Stryker plans to launch a more "user friendly" software upgrade for its hip application in the second quarter of 2020, CEO Kevin Lobo told analysts.
Even as it enjoys returns from its robotic investments, Stryker faces Zimmer Biomet's Rosa platform as the rival system begins to gain traction in knee replacements. Morningstar's Wang called out Rosa's efficient procedure times and design that allows surgeons to use their own instruments as advantages. Zimmer Biomet CEO Bryan Hanson said the company will expand its sales force and surgeon training this year to support Rosa's growth and is aiming for partial knee and hip applications for the robot in 2020.
Not to be counted out, J&J is planning to enter the robotics mix in orthopaedics as well, initially with a portable, low-cost digital surgery system it has dubbed VELYS. The company plans a mid-2020 regulatory submission for the device, Gorsky said in January. Eventually, J&J expects to integrate the technology, which it gained through its 2018 acquisition of Orthotaxy, into one robotics platform.
Medtronic looks to maintain lead in spine as competition develops
Medtronic's Mazor robot, acquired in 2018, provides navigation and robotic guidance in screw placement during spinal surgeries. Surgeons began using the platform in January of last year, and the company has credited the system with spurring demand for its spine implants.
While Medtronic is the early frontrunner in the space, others are preparing to mount challenges, with hopes of stimulating demand in what for years has been a sluggish market in spinal procedures. Stryker, Zimmer Biomet and NuVasive all are working on robotic systems for spine, but when those offerings will hit the market is unclear.
Stryker has said it is pursuing an indication for its Mako platform in the spine segment but has not yet revealed a timeline to market for the application. Zimmer Biomet received FDA clearance in March 2019 for its Rosa system for spine surgeries but is focusing on launching its robotic partial knee and hip applications before it turns its attention to spine, Hanson has said.
NuVasive has also been working on a robotics application for its integrated spine surgery platform, called Pulse. In an update to investors last week, NuVasive execs said the company has been working through "beta evaluations" of the technology for the last few quarters, with plans now to launch the system in 2021.