Dive Brief:
- Apple received 510(k) clearance for a new feature for its smart watch that shows users an estimate of how frequently their heart rhythm shows signs of atrial fibrillation (AFib).
- The Food and Drug Administration cleared Apple Watch for use in the detection of irregular heart rhythms in 2018. The new feature is intended to help patients diagnosed with AFib to track the effect of lifestyle factors on the frequency of their irregular heart rhythm.
- Apple disclosed the new feature shortly before Rune Labs received 510(k) clearance for an application designed for the Apple Watch that uses measurements captured by the wearable to monitor Parkinson’s disease patients. Rune said the clearance will offer particular benefits to patients who use Medtronic's Percept neurostimulator.
Dive Insight:
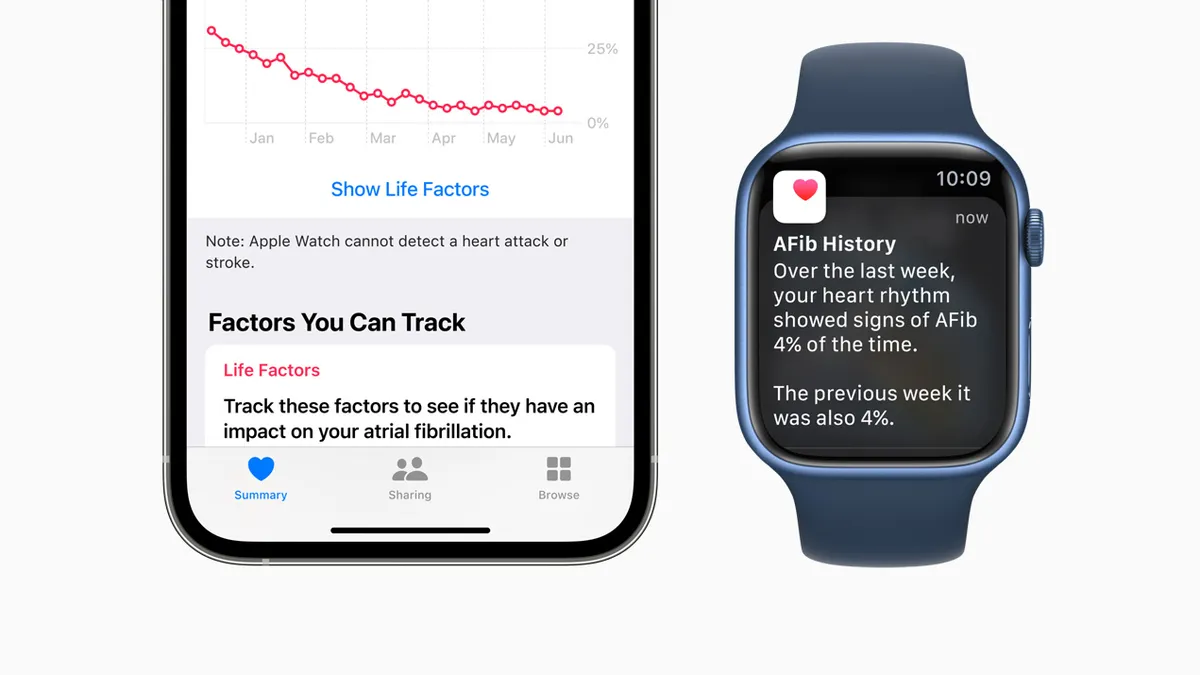
Apple Watch already features an electrocardiogram that alerts wearers to signs of irregular heart rhythms that could potentially indicate they have AFib. The feature offers wearers a route to the detection of the cardiac condition but does nothing to help them manage their condition after they have received a formal diagnosis. Apple now has filled that gap.
“We hear from so many of you who have received an atrial fibrillation alert and sought potentially life saving care,” Sumbul Ahmad Desai, Apple’s VP of health, said at a company event. “So, we also wanted to offer support once you've been diagnosed. When living with this condition, it's important to understand the time you spend in AFib because it may relate to your risk of serious complications such as stroke.”
The American Heart Association published a statement in 2020 arguing that lifestyle and risk-factor modification should become the fourth pillar of AFib management, joining well-established approaches such as anticoagulants. The position reflected evidence that lifestyle changes such as weight loss and physical activity can reduce atrial fibrillation burden.
With the new Apple Watch feature, AFib patients may have an easier way to track the frequency of AFib over time and see whether lifestyle changes may be having positive effects. The new Apple Watch history feature estimates the frequency of AFib and allows users to view lifestyle factors such as sleep, alcohol consumption and exercise that may influence their heart rhythm.
It’s one of two Apple Watch capabilities cleared in quick succession. The second of the clearances covers a third-party app developed by Rune Labs to turn Apple Watch into a device for collecting information on Parkinson’s disease. The app, StrivePD, uses the device’s movement disorder features to monitor tremors and dyskinetic symptoms for transmission to an iPhone app.
For patients who use Medtronic's Percept PC Deep Brain Stimulation device, Rune expects the clearance to “enhance clinicians' ability to make brain-sensing data from these devices useful.”
Medtronic entered into a partnership with Rune late last year.