Dive Brief:
- A research letter and editorial published online Monday in JAMA Internal Medicine renews debate over whether embolic protection devices (EPD) intended to trap plaque debris during transcatheter aortic valve replacement procedures meaningfully lessen stroke risk.
- Authors cite figures from 2017 suggesting that periprocedural stroke still occurs in 2% of patients undergoing TAVR, a growing group considering increasing popularity of the procedure. Whereas Boston Scientific trailed Medtronic and Edwards Lifesciences in introducing its own TAVR device to the U.S. market, Boston's Sentinel device can be used even in TAVR procedures when surgeons are implanting a competitor's valve.
- But an analysis of almost 11,000 TAVR patients showed no statistically significant reduction in stroke between those treated with Boston's Sentinel EPD and those without. In commentary accompanying the research letter, a pair of cardiologists argued that regulatory clearance should be based on clear evidence of patient benefit.
Dive Insight:
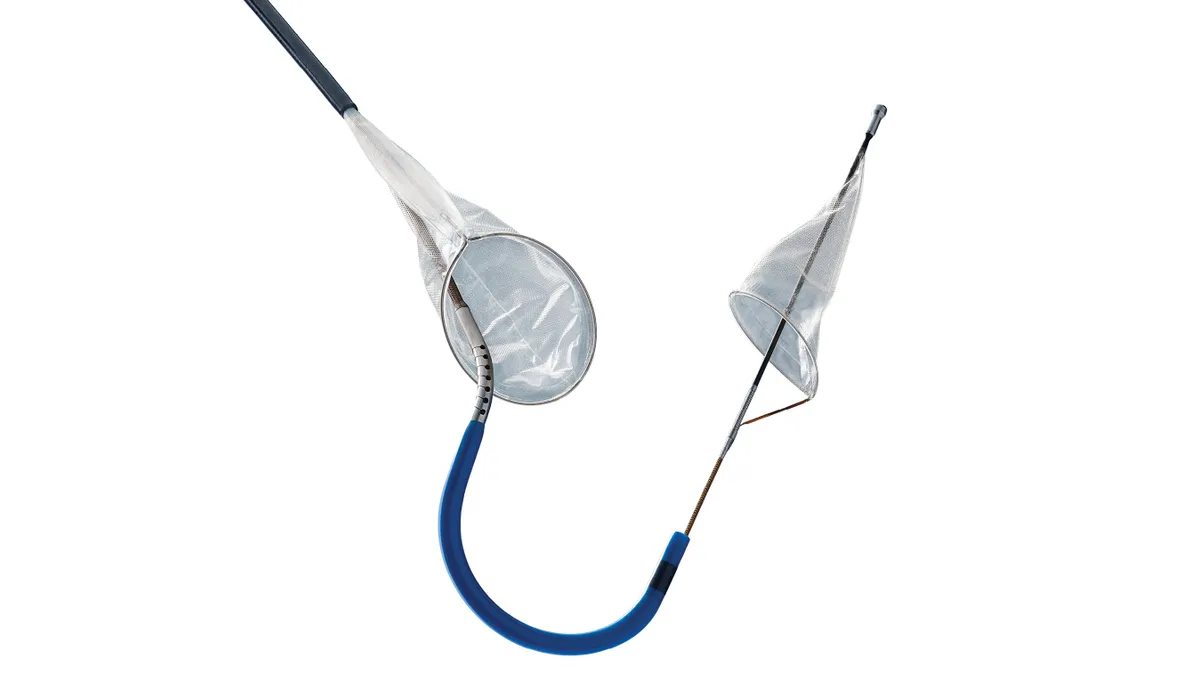
Sentinel received FDA De Novo clearance in 2017 and is the only cerebral EPD on the U.S. market. The device is designed to capture debris in the carotid arteries dislodged during TAVR before it can reach the brain.
According to the JAMA Internal Medicine publications, the proportion of TAVR patients who received EPD during their procedure increased more than six-fold between the third quarter of 2017 and the fourth quarter of 2018, rising from 2.8% to 17.3%.
The articles highlight the uncertainty that persists over the effectiveness of embolic protection devices. Study authors, affiliated with West Virginia University, Mayo Clinic School of Medicine, the Center for Advanced Analytics and Informatics, and Rush University, noted data on the device to date is conflicting.
Their research did not find a lower risk of stroke associated with EPDs in its review of data for 10,985 patients from the Vizient consortium of hospitals. In the 363-person Claret Medical-supported trial that backed FDA clearance for Sentinel, the device did not lower the risk of post-procedure stroke but did catch embolic debris in 99% of filters. Other, smaller observational studies, however, have suggested a benefit to using the devices exists, the research letter said.
Boston Scientific paid $220 million to acquire developer of the technology Claret Medical in 2018. On the same day that the company announced the completed deal, CMS said it would permit a new-technology add-on payment of up to $1,400 for the device, reversing its earlier decision after reconsidering data backing the device’s effectiveness.
In their commentary on the research, cardiologists Rohan Khera and Saket Girotra suggested that FDA’s EPD labeling is “somewhat misleading” because the device is cleared for embolic protection during intracardiac procedures and not specifically for stroke prevention, its intended use. In a sign that the clinical community is not convinced of the device's benefit, they said, EPD was not being used in more than 80% of patients with TAVR and two-thirds of TAVR hospitals by the end of 2018.
In our @JAMAInternalMed commentary, @saketgirotra & I dig into the evidence base for cerebral EPD used in TAVR (Sentinel). We encourage reading the data leading to @US_FDA approval. So often the real-world use vastly exceeds the evidence base. https://t.co/reUBIqjIVs https://t.co/iLn2hjlf7k pic.twitter.com/Tdi1ChWQe3
— Rohan Khera (@rohan_khera) February 25, 2020
"It is imperative that regulatory clearance for clinical devices is based on unequivocal evidence of patient benefit. In the case of cerebral EPDs, efficacy in preventing strokes, not just capturing embolic debris, should be the evidence standard before unrestricted clinical use," the doctors wrote in the editorial. Ultimately, authors recommended further analysis of prospective registries and randomized clinical trials.
The analysis comes as Boston Scientific positions Sentinel as an important growth driver in its structural heart business. On its early February earnings call, the company said the device is being used in over 600 U.S. hospitals and believes it's approaching use in 1 out of 5 TAVR procedures.
CEO Mike Mahoney said the company is beginning enrollment this quarter in a clinical trial for protected TAVR. "We believe that definitive evidence focused on a stroke endpoint will continue to elevate Sentinel to become the standard-of-care for all patients and will help influence future clinical guidelines," Mahoney said.
According to a listing for the trial on ClinicalTrials.gov, the study aims to have 3,000 participants and wrap up during the summer of 2022. The primary endpoint is all forms of stroke within 72 hours of TAVR procedure or time of patient discharge.
Following the journal publication, Boston Scientific said the device "has a robust body of clinical evidence and commercial experience in more than 30,000 patients supporting its use in patients undergoing TAVR," per a statement shared with MedTech Dive Tuesday.
This story has been updated with comment from Boston Scientific.