Dive Brief:
-
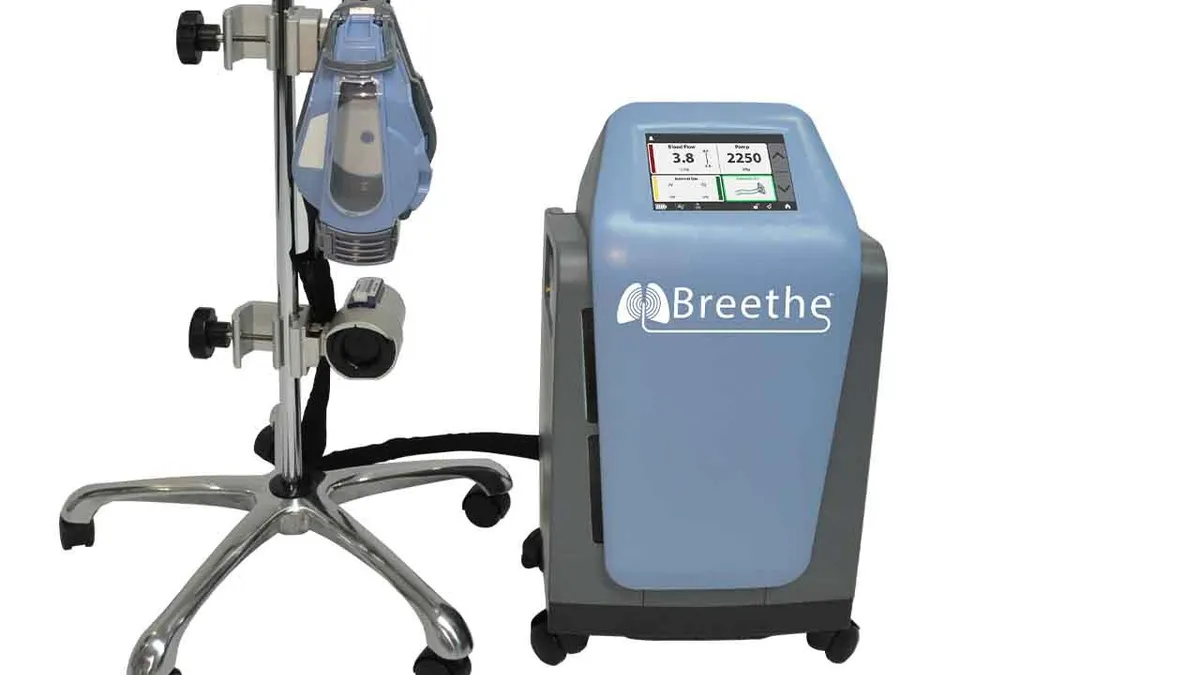
FDA has granted 510(k) clearance to Abiomed’s cardiopulmonary bypass system, setting the company up to treat COVID-19 patients and other people with cardiogenic shock or respiratory failure.
-
Abiomed added the portable extracorporeal membrane oxygenation (ECMO) device to its portfolio in April through the acquisition of Breethe. The deal was underpinned by evidence that Abiomed’s Impella heart pump can complement ECMO in a combination known as ECpella.
-
Having secured the clearance, Abiomed is planning a controlled launch of the system before ramping up to full commercial supply next year.
Dive Insight:
Physicians have used Impella and ECMO in around 10,000 cardiogenic shock patients over the past decade, according to Abiomed, with some evidence that the combination improves rates of heart recovery. The effects of coronavirus infection on the heart and lungs have created a new need for such support.
Abiomed bought Breethe for its portable ECMO device early in the pandemic. In August, Abiomed followed up by securing emergency use authorization for Impella in COVID-19 patients. The EUA cleared physicians to use Impella to provide left ventricular unloading to COVID-19 patients receiving ECMO.
“Unloading itself creates a positive cascade. It rests the myocardium, it helps it to recover. Adding in the need for oxygenation leads to more ECpella discussions because the end goal for all of these patients has got to be survival with native heart recovery,” Abiomed CEO Michael Minogue said on a conference call with investors.
The Breethe 510(k) sets Abiomed up to provide both components of the ECpella combination. Other companies including LivaNova and Medtronic already sell ECMO devices. Abiomed is focusing on the relatively compact, all-in-one design of its system to differentiate the device from the pack. The portability of the system “supports transition from bed to ambulation,” Zachary Kon, cardiothoracic surgeon at New York University, said in a statement.
News of the 510(k) clearance follows a flurry of presentations of data on Impella at TCT Connect, this year's virtual edition of the Cardiovascular Research Foundation’s annual scientific symposium.
One presentation looked at data on the use of Impella RP in right heart failure patients, the indication FDA addressed last year in an alert about a higher-than-expected mortality rate. A subsequent update showed that survival rates were as expected in the subgroup of patients who would have met the enrollment criteria for the pre-authorization trial.
The TCT presentation reaffirmed that Impella RP improves outcomes when used at the right time in the right patients. Researchers linked Impella RP to a 73% survival rate when used within 48 hours of cardiogenic shock onset, compared to 14% in patients who received delayed right-heart support. The result is in line with the data shared by FDA and the pre-approval trial.
Abiomed also used the event to share data from its PROTECT III post-approval study of Impella 2.5 and Impella CP in high-risk percutaneous coronary intervention (PCI) patients, adding to evidence the devices reduce rates of death, stroke, myocardial infarction and repeat procedures.
Other presentations covered the use of Impella before PCI and an interim analysis of a study in high-risk PCI patients. Two analyses of data generated in the RECOVER III post-market study found benefits to implanting Impella before PCI. One analysis showed pre-PCI Impella use has a 74% relative survival benefit in women compared to post-PCI use. The high-risk PCI trial linked Impella to significant improvements in left ventricular ejection fraction and symptoms of heart failure symptoms and angina.