Dive Brief:
- Abbott Laboratories shared results of a postmarket study of 511 people showing its TriClip system benefited patients with tricuspid regurgitation, a condition where the valve between the two right heart chambers does not close properly.
- According to late-breaking data shared at interventional cardiology conference EuroPCR in Paris, 77% of patients who had their valves repaired with Abbott’s TriClip system experienced a reduction of tricuspid regurgitation grade to moderate or less, and 79% achieved New York Heart Association Functional Class I/II, meaning they had no or few restrictions on physical activity. About 2.5% of patients experienced a major adverse event.
- Abbott’s TriClip device is approved for use in Europe and Canada, and the company has submitted it to the U.S. Food and Drug Administration.
Dive Insight:
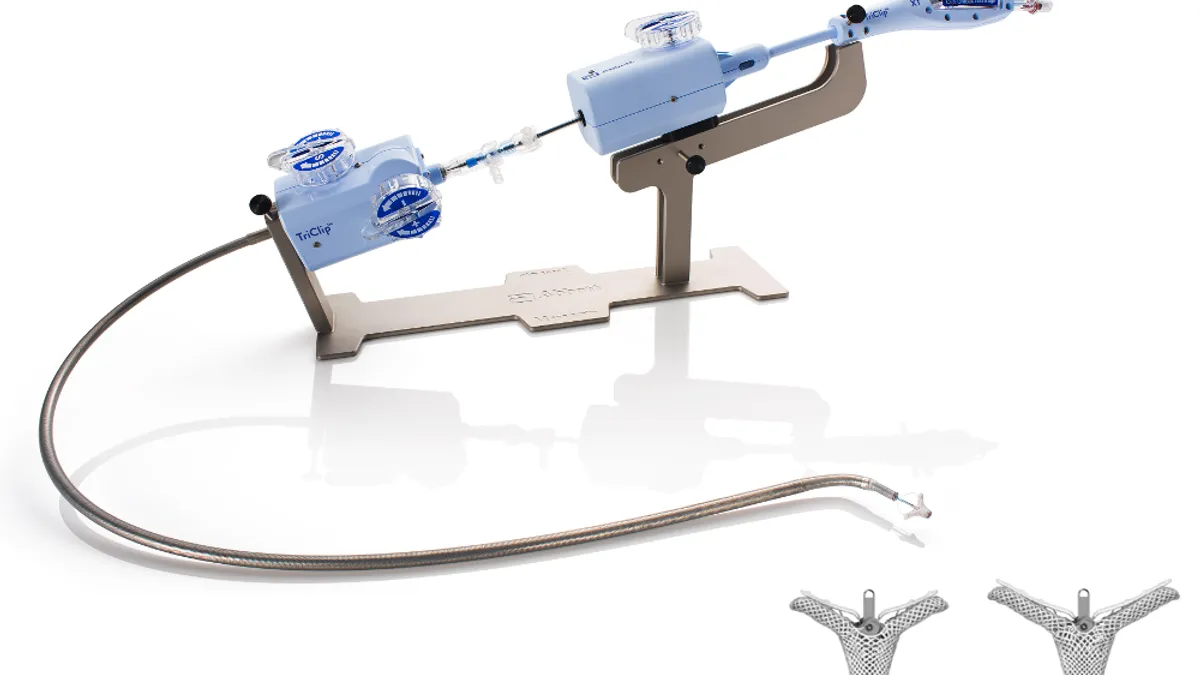
Abbott’s TriClip device is inserted through a minimally invasive surgery and clips together a portion of the leaflets of the tricuspid valve to reduce the backflow of blood.
Competitor Edwards currently sells its own valve repair system called Pascal Precision, which is approved in Europe to treat both mitral and tricuspid regurgitation and in the U.S. for mitral regurgitation. The company has developed another system, called Evoque, which is designed to replace the tricuspid valve and is still an investigational device.
J.P. Morgan analyst Robbie Marcus estimated the market for tricuspid valve repair at 1.6 million patients, smaller than the 4 million to 6 million people with severe mitral valve regurgitation. This could lead to a faster rollout for TriClip when Abbott launches the device, he wrote in a research note in March.
The latest data builds on the results of a pivotal trial of Abbott’s TriClip device, which the company presented in March. That study found TriClip yielded a significant reduction in tricuspid regurgitation grade and improvement in quality of life compared to medical therapy, but there was no difference between the two groups in mortality after one year.
“We think the quality-of-life benefit is meaningful for these very sick patients, and which alone should drive adoption as there are limited alternatives,” Marcus wrote. “However, we think there’s a place for both repair and replacement in this market, and with no mortality benefit, this leaves more room [for] Edwards’ Evoque and other replacement solutions.”
Abbott CEO Robert Ford said he was pleased with the results of the pivotal trial during an April earnings call, adding that the company plans to continue to generate more clinical data as it did with its MitraClip device.
“I don't think it's a one study and that's it,” Ford said, adding that the pivotal trial enrolled patients quickly, which is a good sign for physician acceptance and excitement. “And that's because there's not a lot of good treatment options for these patients,” he said.
Ford said the company has seen good momentum in Europe with TriClip, which contributed to 16% sales growth in its structural heart segment in the first quarter.