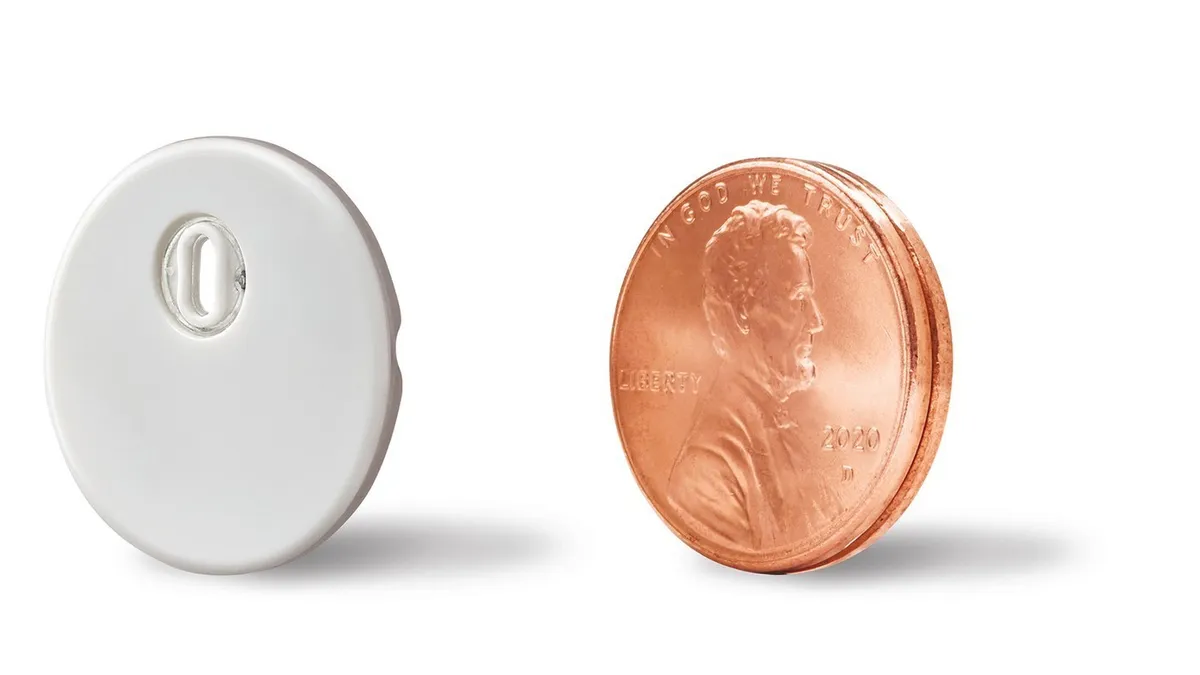Dive Brief:
- Abbott Laboratories said it will invest €440 million ($450 million) in two facilities in Ireland in a bid to increase production of its FreeStyle Libre technology.
- The investment will support the construction of a new manufacturing facility and the expansion of an existing diabetes care site. The new, 250,000-square-foot facility will add capacity for Abbott’s Libre continuous glucose monitoring system.
- The move comes as the company steps up the European rollout of its Libre 3 device, which received a CE mark in 2020 but was initially restricted to a limited launch in Germany.
Dive Insight:
Abbott is facing increased competition from Dexcom in Europe, which received a CE mark for its G7 device in March and began a commercialization strategy that initially targeted large markets such as Germany and the U.K.
Abbott has been expanding its own launch and CEO Robert Ford told investors last month that the company “had very good success in Germany in terms of upgrading the base, and then with the benefits of Libre 3 actually seeing some conversions from competitive systems.”
“Freestyle Libre is the number one product in the world for continuous monitoring and it continues to grow very quickly,” Jared Watkin, Abbott’s senior vice president of diabetes care, told The Irish Times on Aug. 12. “Clearly we need to keep up with that demand from a capacity and manufacturing investment perspective.”
The Irish expansion will give Abbott a new facility in Kilkenny and enhance its existing site in Donegal, creating 1,000 jobs in the process. Watkin said Abbott will start hiring for some of the new jobs immediately, although the new facility remains subject to planning permission and is expected to come online in 2024.