Dive Brief:
- Abbott is recalling a communication system that monitors heart failure patients implanted with its Heartmate 3 left ventricular assist device (LVAD) due to a risk that the system could unexpectedly cause the heart pump to stop or start.
- The issue could have serious adverse health consequences for patients, including lightheadedness, sudden change in blood flow, loss of consciousness, and death, the Food and Drug Administration said Monday in a recall notice.
- Eight patient injuries and no deaths were reported in connection with the problem, the FDA said. The agency labeled the event a Class I recall, noting the correction does not necessitate product removal.
Dive Insight:
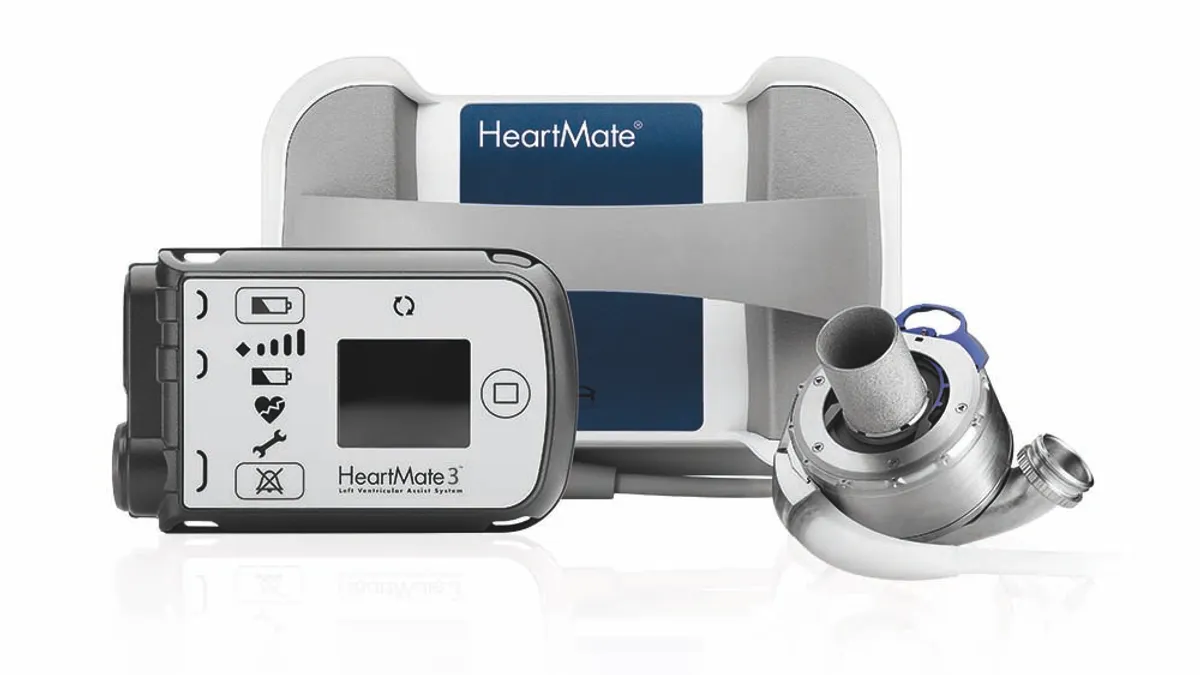
Abbott’s Heartmate device, acquired in the 2017 purchase of St. Jude Medical, provides mechanical circulatory support for patients with end-stage heart failure, sometimes serving as the bridge to a heart transplant.
Abbott is now the only supplier of left ventricular assist devices to the U.S. market, after Medtronic pulled a competing pump in June 2021 following a series of recalls. The FDA advised healthcare providers to stop new implants of Medtronic’s Heartware device, citing a higher frequency of neurological adverse events and mortality compared to other available devices, and complaints that the pump may delay or fail to restart.
The agency told providers to use Abbott’s Heartmate 3 LVAD as an alternative to Medtronic’s system.
Yet use of Abbott’s life-extending device also carries risks. Death, bleeding, right heart failure, respiratory failure, device malfunctions, stroke and heart attack are among the adverse events that may be associated with use of the system.
In an emailed statement Monday, an Abbott spokesperson said Heartmate 3 is safe to use.
“There were no reports of patient harm, the device remains safe to use as outlined in its instructions for use, and Abbott is in the process of developing and releasing a software update to correct the issue,” Abbott spokesperson Shelley Lange wrote in the email.
Abbott is recalling version 1.0.32 of the Heartmate Touch communication system. The system, which works with a controller, is used in hospitals to provide a detailed, large-scale display of a patient’s cardiovascular status during LVAD implant procedures or other situations that require close monitoring.
The suburban Chicago-based company notified physicians on Jan. 3 that it had received a small number of complaints about the controller and an application error that could cause an unintentional start or stop of the Heartmate device, Lange said.
Issues can arise if the communication system gets disconnected from the controller during a “pump stop” command, the FDA said. If the heart pump is running when the communication system is reconnected to a controller, the pump will stop. If the pump was stopped at the time of reconnection, it will restart. There are no alarms to warn users that the “pump stop” command is still in the queue, the agency said.
In its January letter to physicians, Abbott said three of the eight reported issues happened during implant or explant surgery, when extended procedure time is a risk. The remaining five events occurred when patients visited a clinic or hospital.
“Unexpected pump stop during a clinic/hospital visit could result in hemodynamic compromise or syncope,” Abbott said in the letter.
The recall affects 1,560 devices distributed in the U.S. between May 7, 2020, and Dec. 18, 2023.