Dive Brief:
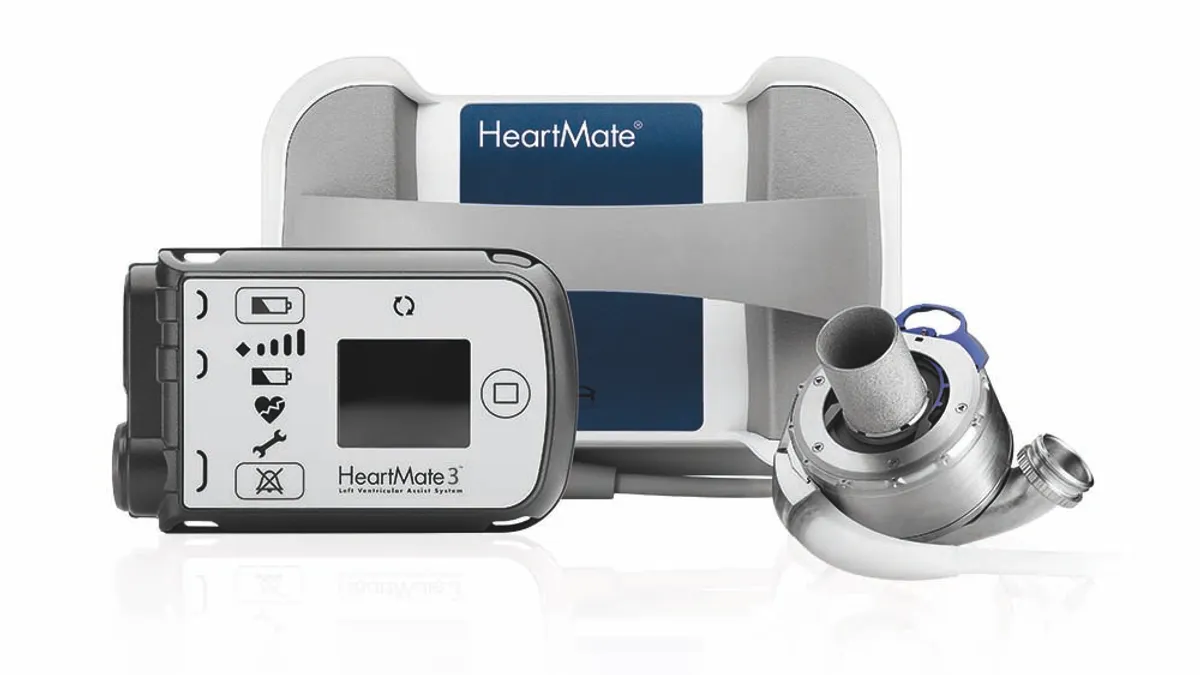
- Removing aspirin from the drug regimen given to people with Abbott’s HeartMate 3 heart pump reduces hospitalizations without increasing the risk of blood clots, according to a randomized clinical trial.
- The study, details of which were published Nov. 11 in JAMA, found patients who took a placebo rather than aspirin had a lower rate of nonsurgical bleeding events with no increase in stroke or other thromboembolic events.
- An accompanying editorial said the “data provide an opportunity to immediately improve the outcomes of patients implanted with contemporary [left ventricular assist devices] (LVADs) by avoiding aspirin therapy as a new standard of care.”
Dive Insight:
Aspirin is part of the recommended anticoagulation regimen for patients who receive LVADs in most treatment guidelines. However, bleeding complications, which could be exacerbated by the antiplatelet agent aspirin, are a problem for LVAD patients. Observational data on patients with Abbott’s older heart pump suggested aspirin can be stopped, but doing so may raise the risk of device thrombosis.
Abbott designed the ARIES-HM3 trial to clear up the uncertainty, randomizing 628 patients with LVADs and advanced heart failure to take aspirin or a placebo on top of vitamin K antagonists. The rate of survival free of nonsurgical hemocompatibility adverse events, the primary composite endpoint, was higher in the patients in the placebo arm, suggesting that aspirin can safely be eliminated from the regimen.
The numerical advantage was driven by a lower risk of major nonsurgical bleeding in the placebo group. For every 100 patients implanted with an LVAD, eliminating aspirin prevented 14.5 major bleeding events in the first year. Stroke and other thrombotic events were rare across the trial, but again the numbers favored the placebo group. Abbott estimated a 41% reduction in costs related to bleeding events.
In a statement, Robert Kormos, divisional vice president for global medical affairs at Abbott's heart failure business, framed the results as a challenge to the consensus that aspirin should be part of the treatment for heart failure patients with an LVAD. Michael Felker, a cardiologist with the Duke Clinical Research Institute, and Joseph Rogers, CEO of The Texas Heart Institute, concurred in a JAMA editorial published alongside the paper.
“The results of the ARIES-HM3 trial appear robust and convincing as to the lack of benefit from aspirin therapy in most patients receiving support from an LVAD,” the editorial authors wrote. “For patients who meet the inclusion and exclusion criteria for this trial and are undergoing implantation of an LVAD, these data suggest that aspirin therapy can be avoided to limit bleeding complications and optimize outcomes.”
While arguing that avoiding aspirin should be the new standard of care, the authors identified areas of uncertainty that need additional data, such as whether aspirin should be stopped in patients who are taking the drug without bleeding complications, and how to treat people who have had a thromboembolic complication from the support device.