Dive Brief:
- Abbott received Food and Drug Administration clearance for a reader for its newest continuous glucose monitoring (CGM) system.
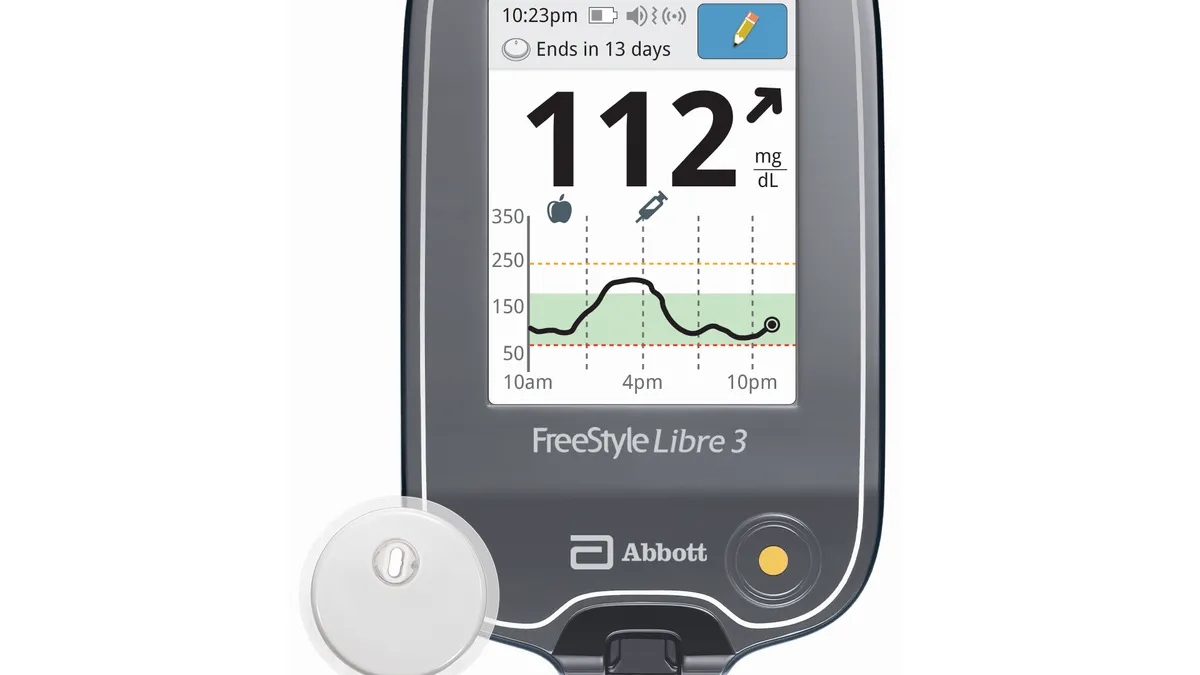
- Its FreeStyle Libre 3 system received clearance in May 2022, but at the time, monitoring could only be done through a smartphone.
- Abbott was expected to eventually release a reader for the system, as having a separate reader is required for Medicare reimbursement, RBC Capital Markets analyst Shagun Singh wrote in a Sunday research note.
Dive Insight:
Since Medicare covers CGMs as durable medical equipment, the devices must include a component, such as a reader, that is expected to last at least three years. Now that Abbott’s Libre 3 reader is cleared, the company said it is working to get the CGM added to Medicare’s list of covered systems “as soon as possible.”
To explain the importance of Medicare coverage, Singh noted one doctor was putting all of her new CGM patients on Dexcom’s G7 because Abbott’s device is not covered by Medicare.
“She mentioned that wouldn't be the case if reimbursement comes more quickly, especially because she likes the Libre platform,” Singh wrote.
It’s also critical as Medicare coverage of CGMs expands this year to include people using all types of insulin, and people with low blood sugar events. The reimbursement expansion went into effect on Monday.
Going forward, the Libre 3 system can work with a smartphone app or a separate reader. Recently, Abbott recalled the readers for its FreeStyle Libre CGMs, which it said could catch fire if improperly stored or charged. Abbott said the adverse incidence rate is very low and only occurred from improper reader use, Singh wrote. The company is continuing to sell readers and offer free replacement devices to users who experience an issue.
Abbott claims some 4.5 million people in 60 countries use its FreeStyle Libre products to help manage their diabetes, making it the most widely used set of diabetes devices in the world.