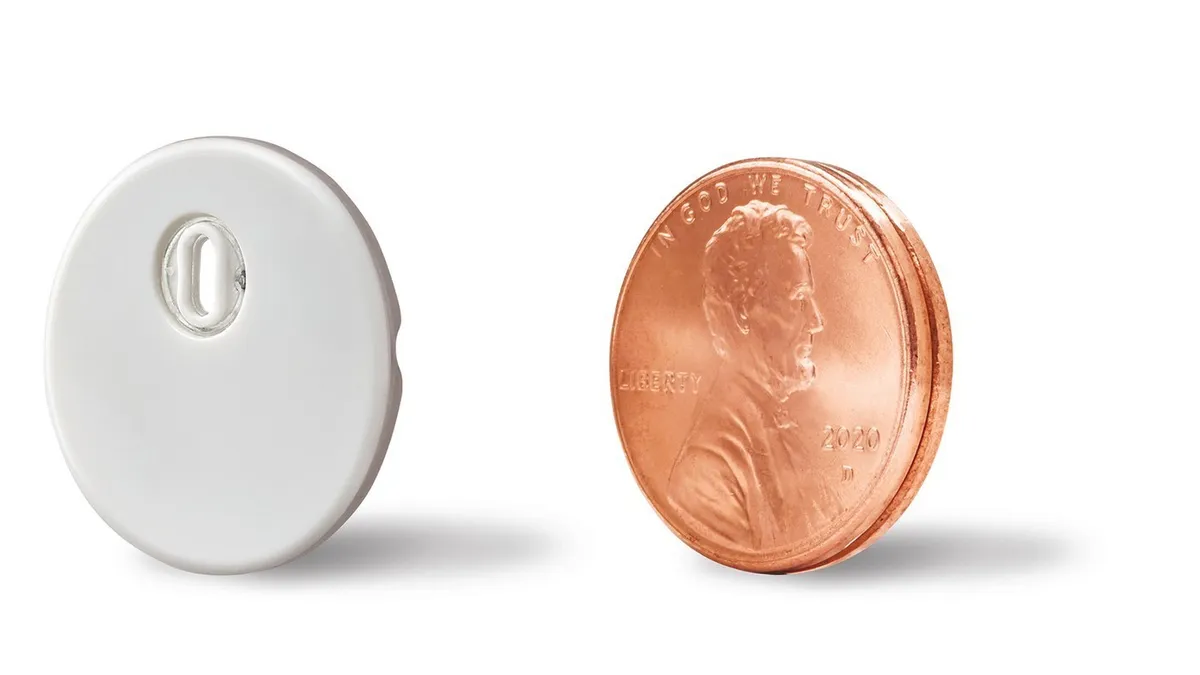Dive Brief:
- Abbott's latest version of its FreeStyle Libre glucose monitoring device for diabetes management has received a CE mark and will be made available in "the coming months," the company announced Monday.
- European approval for the smaller and thinner third-generation sensor comes just over three months after Abbott received a drawn out U.S. clearance for the second-generation version of its device. It's been almost two years to the day since Abbott announced a CE mark for FreeStyle Libre 2.
- Meanwhile, Dexcom has yet to roll out the most recently developed version of its CGM, dubbed G7, which also has a much smaller sensor. Disruptions from the pandemic pushed back the soft launch of the product.
Dive Insight:
The regulatory OK for Abbott is the latest development in the rivalry between the two big continuous glucose monitor players, which sell-side analysts on Wall Street described as helping it close its technology gap with Dexcom. Either way, analysts contend the CGM market is so undertapped that there remains significant runway for both companies.
SVB Leerink analysts called the Libre 3 progress "a meaningful step forward in closing the technology gap" with competitor Dexcom, but emphasized that the CGM market is currently a "rising tide lifts all boats" scenario. They said in a note to investors Monday, "we don’t view this as a competitive market share grab story but rather a [total addressable market] expansion story."
Analysts at Cowen noted that while Abbott did not disclose accuracy data for Libre 3, they expect more to be shared in early 2021. And the addition of real-time alerts should in the future enable the device to integrate with automated insulin delivery systems from Tandem and Insulet, the analysts said, likely by the end of 2021 or early in 2022.
The 14-day wear Libre 3 sensor marks the first time Abbott has updated the sensor design since the original FreeStyle Libre rollout in 2014, an Abbott spokesperson clarified. The change reduces the sensor size by 70% and the company claims it now sells the smallest, thinnest CGM sensor available.
As for functionality, Libre 3 allows for continuous, automatic data transmission to a smartphone every 60 seconds, removing the previous need to manually scan the sensor with a device, which made the system more of a flash glucose monitor.
Although Abbott only recently received the U.S. green light for Libre 2, the company did post information on a U.S. study of Libre 3 to ClinicalTrials.gov in July. That 100-participant study appears to not yet be recruiting and has not been updated since July. On Monday, an Abbott spokesperson said the company does not have "any further details to share at this time on FreeStyle Libre 3 in the U.S.," and that the company is currently just focused on expanding Libre 2 within the U.S.
Also in Europe, Abbott earlier this month launched a novel iteration of the system specifically for athletes interested in closely tracking their blood glucose levels, broadening the product line for the first time to people without diabetes.
Abbott said it's keeping pricing for the new version of the device the same as earlier iterations of the system. While the cost of the therapy may vary by a user's insurance or point of access, the Abbott system is widely regarded as a more affordable alternative to Dexcom's products.
A Dexcom spokesperson said Monday that the company would not comment on G7 again until its next earnings call in late October, but reiterated from an investor call ealrier this year that G7's hardware, sensor and electronics design, as well as the algorithm, are all locked in and complete.