Dive Brief:
- Continuous glucose monitor market giants Dexcom and Abbott on Wednesday independently announced plans to integrate their newest technologies with a forthcoming automated insulin delivery system from Insulet.
- Called Horizon, the system is currently being assessed in a pivotal trial and Insulet expects to begin selling the device in the second half of this year. The smartphone-based insulin dosing algorithm will use readings from a CGM to direct insulin delivery from a tubeless, wearable pod.
- The news comes as the Advanced Technologies & Treatments for Diabetes conference gets underway in Madrid, where other players like Medtronic and Tandem are also taking part.
Dive Insight:
As interoperability takes off in diabetes — the idea that insulin users should be able to seamlessly pair their preferred devices for measuring blood sugar and administering insulin — a rising tide appears to be lifting all device makers' boats. Uptake of insulin pumps spurs adoption of CGMs, devices that theoretically help insulin pumps work better, and vice versa.
The dual Insulet announcements Wednesday is not the first time Dexcom and Abbott have shared commercial partners. Tandem, Novo Nordisk and Livongo are all examples of companies working to leverage relationships with users of Dexcom CGMs and Abbott's FreeStyle Libre devices alike. Abbott's other partners include Bigfoot Biomedical, Sanofi and Omada, while Dexcom's list includes Companion Medical, Eli Lilly, Beta Bionics and Verily.
With the deals, Insulet will gain access to some of Dexcom's estimated 650,000 active customers and Abbott's 2 million FreeStyle Libre users.
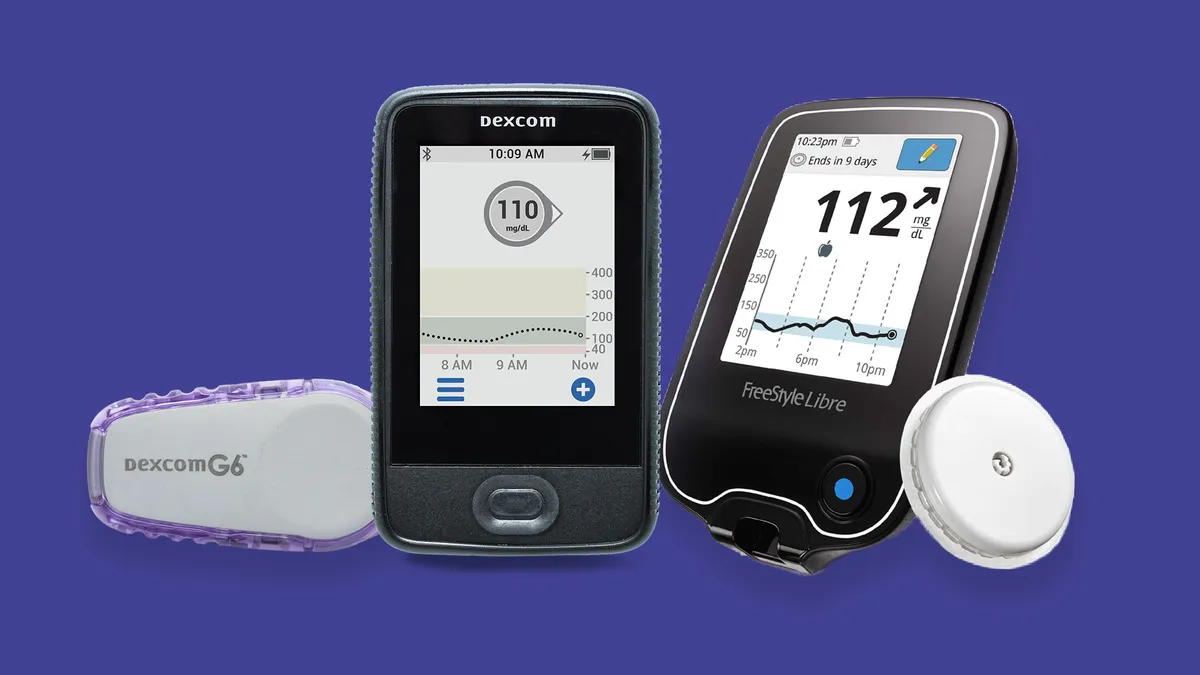
Dexcom's G6 was already compatible with Insulet's Omnipod insulin pump. This latest announcement solidifies plans for Horizon to incorporate Dexcom's widely available G6 CGM, and also confirms that Dexcom's forthcoming technology, called G7, will be compatible with Horizon. G7 is slated for a full rollout in 2021.
Abbott said its FreeStyle Libre 2 device, authorized in Europe but not yet in the U.S., will also work with Horizon. Abbott announced the CE mark for Libre 2 in October 2018. Compared to the earlier version of the device, Libre 2 adds the option to enable alarms signaling high or low blood glucose.
Some analysts have speculated that FDA's long review of Libre 2 is because Abbott applied for iCGM designation, which indicates a CGM can be interoperable with an alternate controller enabled (ACE) insulin pump, as well as an interoperable automated glycemic controller algorithm. An iCGM, ACE pump and the aforementioned controller can together constitute an FDA-authorized, automated insulin dosing system.
The agency may have questions about how well Libre 2 meets the specific iCGM criteria. But the fact that Abbott announced another interoperable insulin delivery partnership, following October plans to integrate with Tandem's technology, suggests further confidence that Abbott will be awarded the iCGM designation, analysts at Stifel wrote in a note to investors Wednesday.
Tandem, which is working on future integrations with Abbott and whose technologies are G6-compatible, has a bit of a head start over Insulet. At the end of 2019, Tandem received FDA's De Novo authorization for its interoperable insulin dosing algorithm called Control-IQ.
Insulet is slated to speak to investors on an earnings call Feb. 25. Last week, the company announced a medical device correction regarding a potential glitch with the personal diabetes managers, or handheld controller devices, used in its Omnipod Dash system.