Dive Brief:
- Tandem Diabetes Care’s artificial pancreas has improved time in blood sugar range in young children with Type 1 diabetes in a clinical trial, according to a paper published in the New England Journal of Medicine.
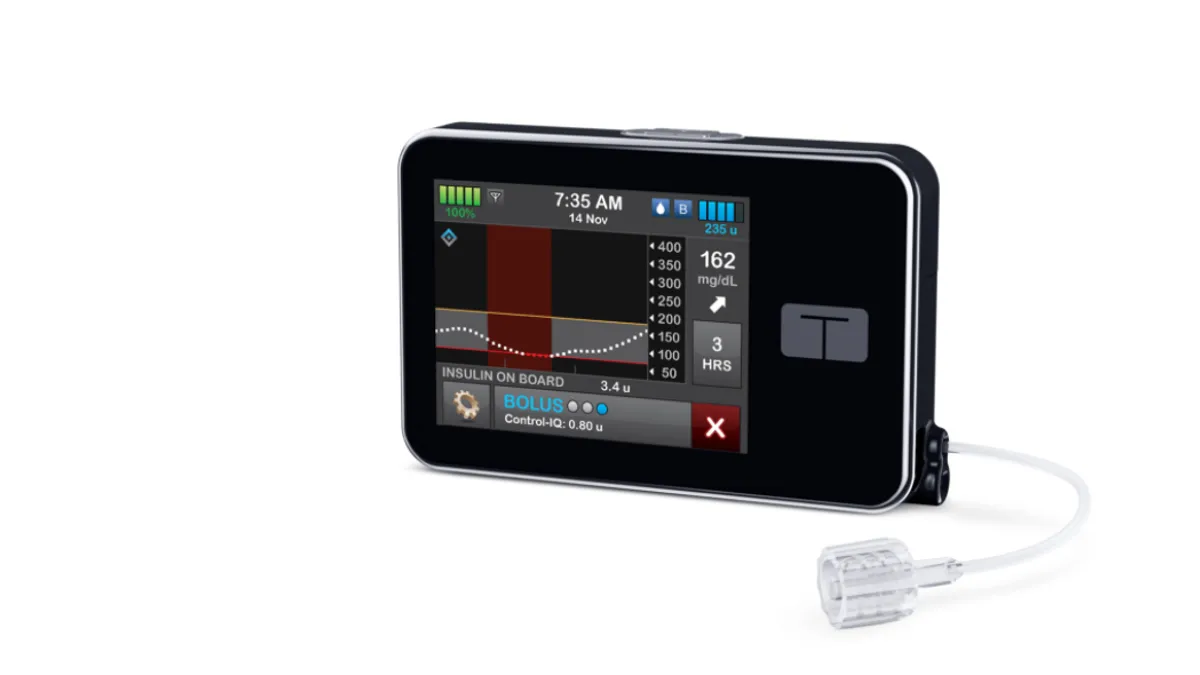
- The study randomized 102 kids ages two to five years to receive multiple daily injections or a closed-loop system, consisting of Tandem’s t:slim X2 insulin pump and Control-IQ technology and Dexcom’s G6 continuous glucose monitor (CGM). After 13 weeks, time in range improved by three hours a day in the closed-loop group and was largely unchanged in the control cohort.
- Tandem’s pump and Control-IQ technology are currently indicated for use in people ages six years and up. Medtronic’s MiniMed 770G System and Insulet’s Omnipod 5 are indicated for kids as young as two years.
Dive Insight:
Young children pose unique challenges for diabetes management that make it harder to meet glycemic targets. Compared to older people, they receive smaller doses of insulin, have less predictable food intake, perform more unscheduled exercise and are less able to articulate the need for treatment of low blood sugar.
Hybrid closed-loop systems can address some of the issues by automating the delivery of insulin based on the analysis of CGM data. Medtronic, which received approval in the U.S. in 2020, was the first to bring such a system to market for use in infants and now competes with Insulet for the opportunity.
In 2021, Tandem and the National Institute of Diabetes and Digestive and Kidney Diseases initiated a study of the t:slim X2 insulin pump and Control-IQ technology in children ages two to five years. Now, the collaborators have published the results of the study.
Time in range for participants in the closed-loop cohort increased from 56.7% to 69.3% over the course of the 13-week trial. The increase works out to an additional three hours a day in the target range. In the control cohort, time in range increased from 54.9% to 55.9%. The increase in the closed-loop group was significantly larger, causing the study to hit its primary endpoint.
Most, 81%, of the participants and caregivers underwent virtual training on the use of the closed-loop system. Dr. Jordan Pinsker, medical director at Tandem, identified that fact as a differentiator for the system in a statement, noting that “automated insulin delivery can be very daunting for parents” and a system “that is simple enough to start with virtual training makes the t:slim X2 insulin pump an excellent therapeutic option for this population.”
The statement lacks details of the next steps for plans to make the system available to the population. In a financial report published last month, Tandem said it had “recently completed clinical studies to support expanding the indications of our Control-IQ technology to include people with type 1 diabetes ages 2 to 5 years old” without providing a regulatory timeline.