Dive Brief:
- Scientists have used 3D printing to create custom, pumping replicas of patient hearts that could enable physicians to test which device fits best before surgery.
- Writing in Science Robotics, researchers from Massachusetts Institute of Technology describe the 3D printing of replicas based on medical scans that mimic the mechanics and physiology of each individual’s actual heart.
- A retrospective study showed the model predicted the hemodynamic changes seen in recipients of transcatheter aortic valve replacement devices, specifically Medtronic’s Evolut R and Edwards Lifesciences’ Sapien 3. The scientists see a role for the replicas in testing TAVR devices.
Dive Insight:
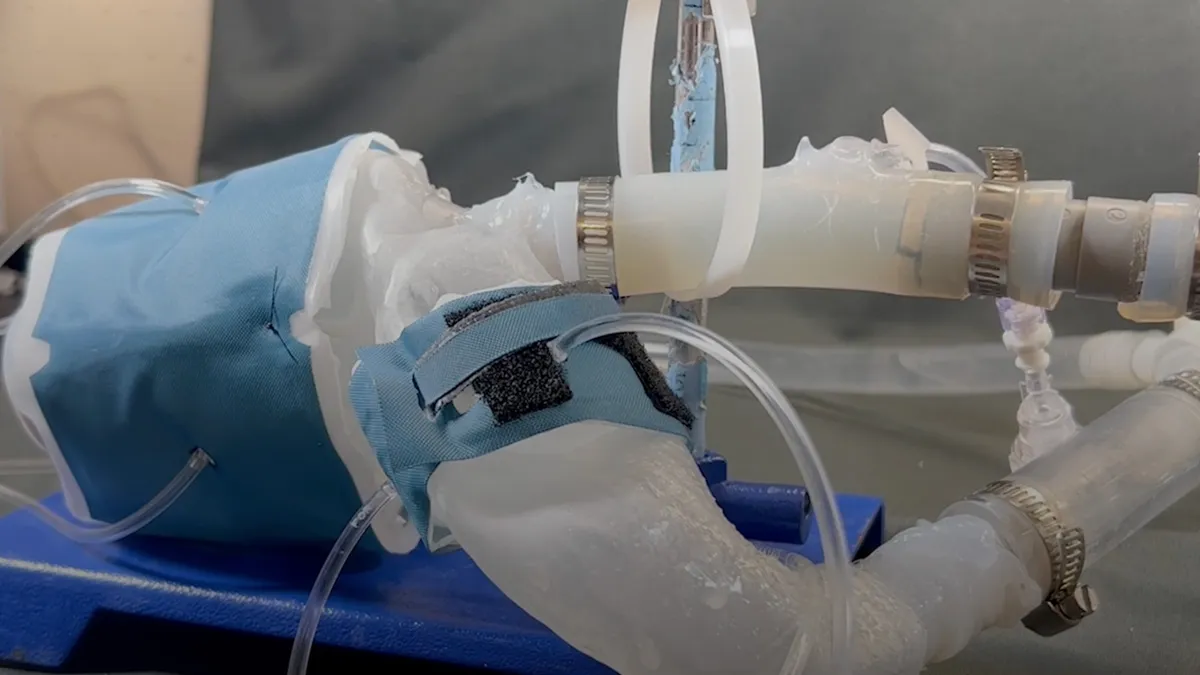
Other groups have used 3D printing to create anatomical models of calcified valves. However, the MIT researchers say differences between the mechanical properties of the materials used in commercial 3D printers and those of native heart valves “greatly compromise” the ability of replicas to reliably mimic patient-specific hemodynamics.
The Science Robotics paper describes an attempt to replicate cardiac mechanics and physiology. Using a polymer-based ink, the collaborators printed a soft, flexible shell in the shape of each patient’s heart and an accompanying custom aorta. Then, the team made patient-specific soft robotic sleeves, with a similar mechanism to blood pressure cuffs, to contract the heart and mimic the pumping of blood.
Christopher Nguyen, assistant professor in medicine at Harvard Medical School and co-author of the paper, outlined how the system could optimize TAVR procedures by enabling physicians to pick the valve that best fits the patient’s anatomy and to plan their procedures.
“Patients would get their imaging done, which they do anyway, and we would use that to make this system, ideally within the day,” Nguyen said. “Once it's up and running, clinicians could test different valve types and sizes and see which works best, then use that to implant.”
The researchers also see a role for the technology in the development of new devices. By testing their devices across a spectrum of clinical cases, Edwards, Medtronic and other developers of TAVR devices could broaden the usability of their products to include patients with unusual anatomies that are a poor fit for existing valves.