Dive Brief:
- A watchdog report found that durable medical equipment suppliers accounted for the majority of providers who had their Medicare enrollment revoked for ineligibility after pandemic flexibilities ended.
- In 2020, the Centers for Medicare and Medicaid Services (CMS) issued temporary waivers to increase workforce capacity and ensure access to services during the pandemic, including postponing fingerprint-based background checks and postponing site visits. The number of new suppliers of durable medical equipment, prosthetics and orthotics increased in pace during this time.
- This group of suppliers accounted for 83% of the 208 enrollment waivers that were later revoked by CMS after finding they were ineligible, according to a Monday report from the U.S. Government Accountability Office.
Dive Insight:
From March 2020 to March 2022, about 2.6% of all newly enrolled durable medical equipment (DME) suppliers were later revoked for being ineligible. This is significantly more than other provider types, which had less than 0.1% of their enrollments revoked, according to the report.
“While this is not a large share of enrollments, even a small number of providers can cause significant financial harm if they commit fraud,” the GAO noted in a summary of its audit.
In July of 2022, the Department of Justice brought charges against 36 defendants for more than $1.2 billion in an alleged fraud scheme that involved telemedicine companies arranging for medical professionals to order expensive genetic tests and medical equipment, regardless of whether patients needed them.
Since new DME suppliers are considered to pose a high risk for fraud or waste, they typically face more scrutiny from regulators. Between March and July of 2020, when some of the screening requirements were temporarily waived, the number of new DME providers increased compared to usual monthly enrollment rates, according to the report.
Medicare enrollment of new DME suppliers increased during pandemic flexibilities
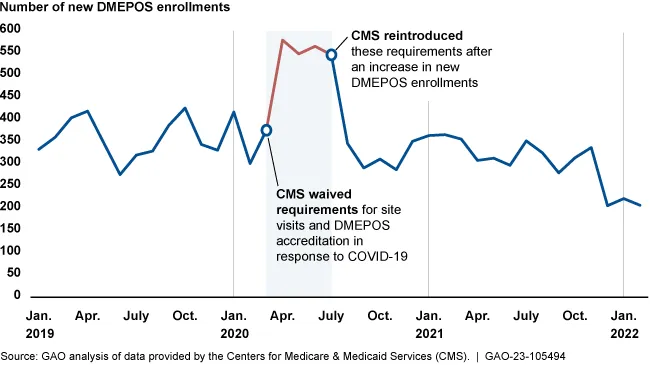
CMS reinstated requirements for site visits and accreditation of DME suppliers in July of 2020, and started requiring fingerprint-based criminal background checks again in October of 2021. For companies that enrolled during the waiver period, CMS intended to perform background checks later, but hasn’t done it yet, according to the GAO.
The watchdog also raised concerns that CMS might not be able to complete 237,000 postponed revalidations of provider eligibility before the next batch of assessments are due.













