Dive Brief:
- A recommendation by the European Society of Cardiology could open a new market for treating medication-resistant high blood pressure with renal denervation.
- Medtronic, which has a CE marked renal denervation device in Europe, said it was “encouraged” by the update in a Thursday statement. ReCor Medical has a competing device in Europe. No such treatments are currently approved in the U.S.
- Previously, renal denervation was only an investigational treatment in Europe, according to a consensus statement from 2018. The guidelines were updated after several trials provided new evidence.
Dive Insight:
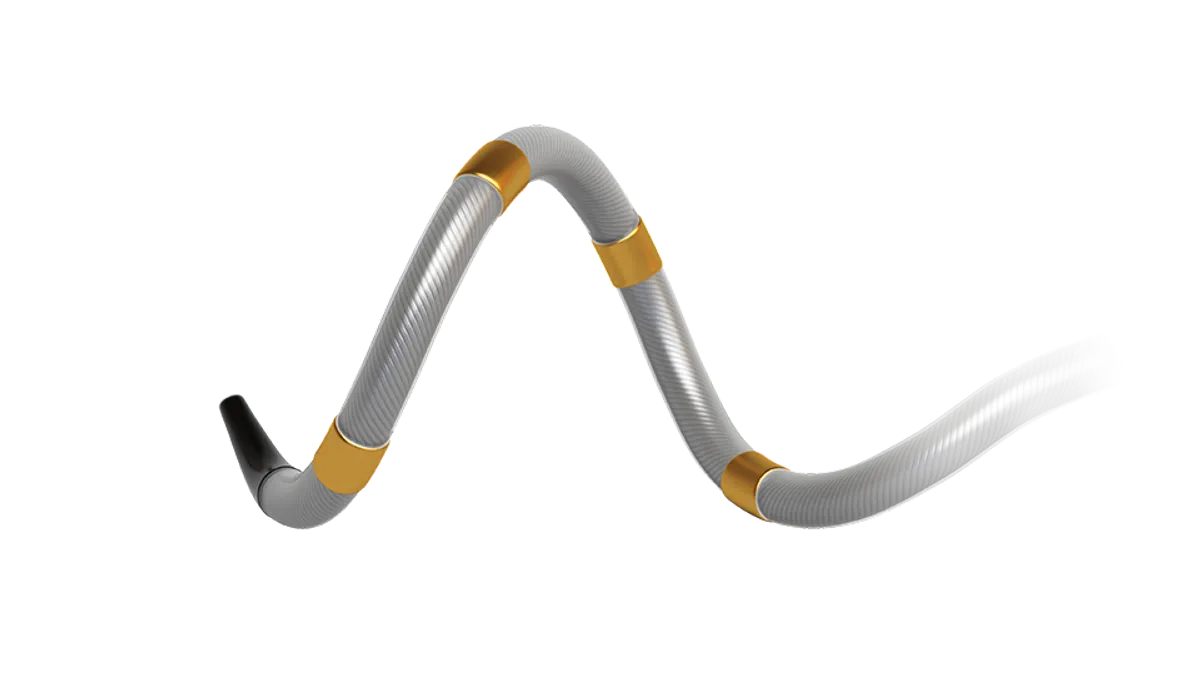
Until now, patients whose high blood pressure persisted despite medication and lifestyle changes had few options. Now, according to a consensus statement published in the European Heart Journal, these patients can be treated with renal denervation, a minimally invasive procedure that uses a radiofrequency or ultrasound device on a catheter to ablate nerves in the renal artery.
Early on, clinical trial failures loomed over the treatment, but results of more recent randomized controlled trials point to safety and efficacy.
Based on these findings, an expert panel recommended the treatment as an option for patients whose high blood pressure hasn’t responded to lifestyle changes and taking a combination of three blood pressure medications. It is also a viable option for patients who can’t tolerate long-term therapy using antihypertensive medication.
It could be used as an earlier intervention if patients “express a strong preference” for renal denervation after “intensive counseling” with a physician, according to the consensus statement.
In the review of results, the experts noted no specific safety concerns other than exposure to radiation and a potential complication related to the procedure to access the artery, which occurred in fewer than 1% of cases.
In the long-term, patients could develop artery stenosis related to vascular injury, and worsening kidney function, they wrote.
The consensus statement should support insurance coverage for the procedure in Europe. Medtronic’s Simplicity Spyral renal denervation system has had a CE Mark since 2013, but insurance coverage had been a challenge, because it was only recommended for use in a clinical trial setting in the past.
In the U.S., the company has submitted results of its recent SPYRAL HTN-ON MED trial to the Food and Drug Administration, with hopes of securing approval this year.