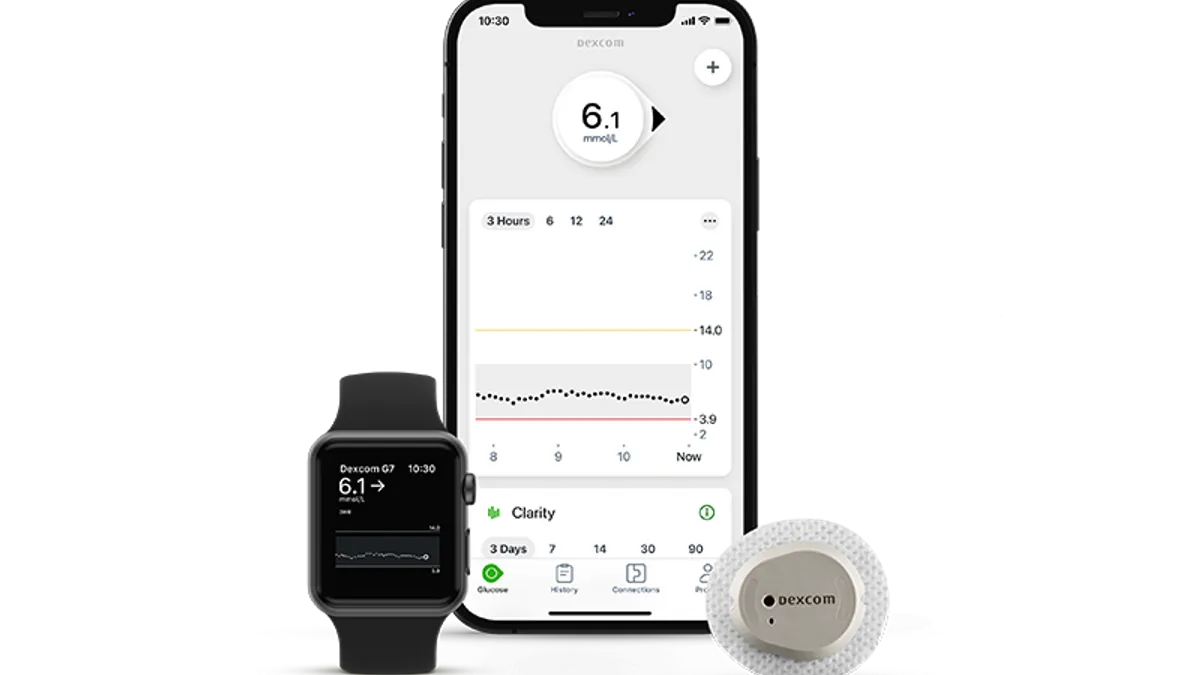
Dexcom’s G7 continuous glucose monitor, which the San Diego-based company expects will be its flagship device, is scheduled to launch early next year after receiving clearance from the Food and Drug Administration. The device received an expanded label, meaning it can be used by people with Type 1 and Type 2 diabetes as well as people with gestational diabetes.
To help ensure the commercial success of the device, Dexcom has been working to make certain that insurers will include the G7 in their coverage and that the company has enough devices to meet what it expects will be a large demand, Chief Operating Officer Jake Leach said in an interview.
Dexcom plans to roll out sales of the G7 “across the globe” in 2023.
In Europe, where the G7 launched in September, the company says it has been seeing strong demand for the device. So far, more than 60% of patients who are starting on the G7 are new to Dexcom, meaning they either hadn’t used a CGM before or switched to Dexcom from a different brand.
Insurance coverage
In the U.S., the company was in discussions with insurers before receiving g clearance, but with the green light from regulators, “we can start to lock coverage in,” Leach said. The COO expects “good” insurance coverage when the G7 reaches the marketplace, but at the outset, “not everyone’s coverage will be there.”
In the interim, Dexcom will allow patients to pay cash for the G7, or to start on an insurance-paid G6 device, then switch to a G7 once the new device is covered. Most of Dexcom’s customers pay for their devices through their insurance’s pharmacy channel, while some patients have coverage through durable medical equipment plans, Leach said.
Currently, Medicare and most private insurers cover the CGMs for patients who are in intensive insulin therapy, where multiple daily injections or an insulin pump are used to manage blood glucose levels. That could expand under proposed new draft coverage guidelines issued by the Centers for Medicare and Medicaid Services that would cover CGMs for patients who are treated with insulin or “have a history of problematic hypoglycemia,” potentially opening the devices to more patients with Type 2 diabetes.
A final coverage decision is expected next year.
“It’s a big deal, a large increase in folks who have access,” Leach said. “What happens is Medicare is generally the first mover, then we’ll see most of the private companies usually follow.”
Compared to traditional glucometers, CGMs can significantly reduce blood glucose levels in patients with Type 2 diabetes according to studies run by Dexcom.
While analyst Margaret Kaczor with William Blair in Chicago has welcomed the G7 as “the definitive form factor for DexCom,” she warned the company is “highly dependent” on new patient growth in an increasingly penetrated market. Competitors Medtronic and Abbott remain “heavily invested” in the CGM market and take a share of existing or new patients, Kaczor wrote in a research note.
Supply chain preparations
As it prepares for the launch, Dexcom is opening a new manufacturing plant in Malaysia, where the G7 will be built. Currently, all of its devices are manufactured in San Diego and Arizona.
Supply constraints have been a recurring problem for device manufacturers since the start of the pandemic, but Leach didn’t expect that it would be an issue with the G7.
“We got way ahead of that because we knew the G7 was coming,” the COO said. “Our teams have done a good job securing the raw materials we need and the capacity to manufacture the products.”
Correction: This article has been updated to correct COO Jake Leach’s first name in the headline.