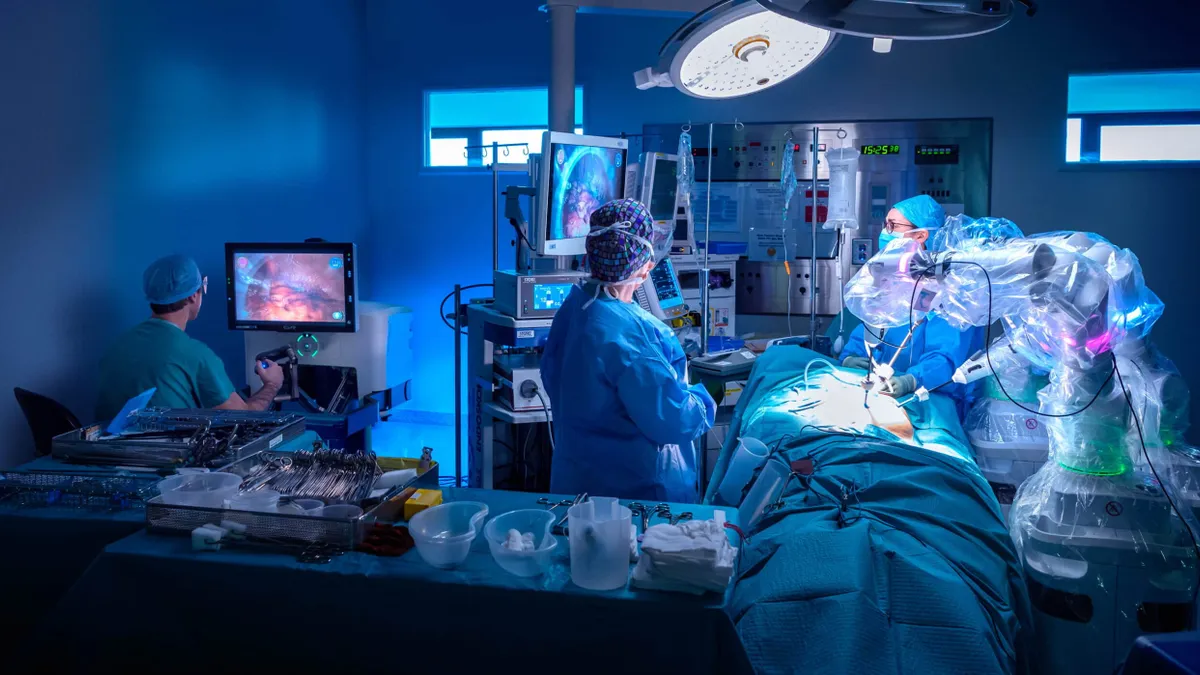
Dive Brief:
- CMR Surgical won Food and Drug Administration authorization for its Versius robot with an initial indication for gallbladder removal surgery. CMR will partner with select hospitals as the first part of a multistage strategic plan to introduce the robot in the U.S.
- The authorization is the first granted through the FDA’s de novo pathway for a multiport, soft tissue general surgical robot, CMR said in a Monday announcement. The de novo process brings new medical devices to market that may serve as predicates for other 510(k) submissions.
- The company announced the milestone less than a week after it named Massimiliano Colella as interim CEO, replacing Supratim Bose, who stepped down for personal reasons after less than two years in the job.
Dive Insight:
Cambridge, U.K.-based CMR is challenging the dominance of Intuitive Surgical in robotic assistance for soft tissue procedures. CMR says that the compact and portable design of its robot makes it flexible enough to be used in multiple care settings.
Versius received Europe’s CE mark in 2019 and is the second-most-utilized robotic surgical system outside of the U.S., according to CMR. More than 26,000 cases have been performed using the system, in Europe, Latin America, Asia, the Middle East and Africa, the company said.
“Versius securing FDA clearance is an important step forward for helping hospitals of any size to be able to offer robotic-assisted surgery,” Erik Wilson, vice chair of surgery at the University of Texas McGovern Medical School at Houston, said in CMR’s statement.
After establishing a global presence, the 10-year-old company has set its sights on the U.S. It sees the robotic surgery market as underpenetrated, pointing to a study showing that about 2.5% of nearly 10 million annual major operating room procedures in the U.S. in 2017 were robot-assisted.
“The U.S. is an important strategic market so gaining FDA authorization for Versius for use in cholecystectomy procedures in adult patients is a significant step forward in CMR achieving its mission of bringing minimal access surgery to more patients around the world,” said Colella, who was CMR’s chief commercial officer before being appointed chief executive.
In recent months, CMR established a leadership team that includes Daniel Moore, former chairman of Livanova, who was named non-executive chairman of CMR’s board. Markus Bauman, previously at Atomico Expert Network, was hired as chief legal and business affairs officer, and Michelle Paknad, from Smith & Nephew, came on board as senior vice president for global business development.
To fund its ambitions, the privately held company raised $165 million in financing last year, on top of a $600 million funding round in 2021.