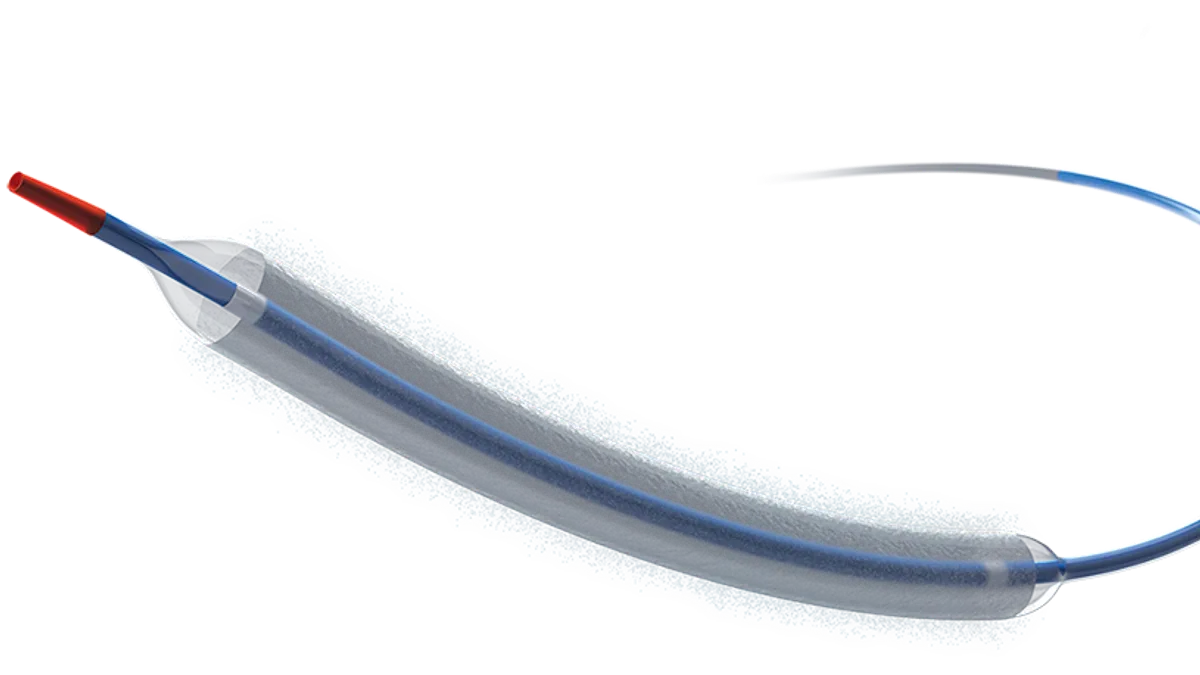
Dive Brief:
- Boston Scientific said Friday it has received Food and Drug Administration approval for a drug-coated balloon to treat coronary in-stent restenosis, a condition in which a vessel that previously received a stent narrows again due to plaque or scar tissue.
- Approval for the device, which received FDA’s breakthrough designation in 2021, was supported by positive results from the prospective, randomized Agent trial that enrolled 600 patients at 40 U.S. sites, the company said.
- “We view Agent highly favorably for its statistically significant superiority in what is a difficult-to-treat and common [in-stent restenosis] patient population, which we think could enable rapid physician adoption,” BTIG analyst Marie Thibault wrote Friday in a note to clients.
Dive Insight:
In-stent restenosis occurs in about 10% of U.S. patients who receive a percutaneous coronary intervention procedure to open clogged heart arteries, according to Boston Scientific.
The Agent drug-coated balloon offers an alternative to traditional approaches to treat the problem such as balloon angioplasty, adding more layers of stenting, or radiation. The balloon catheter is coated with the drug paclitaxel and transfers a dose to the vessel wall to help prevent it from narrowing again.
"The AGENT DCB addresses a critical unmet need by providing a dedicated treatment option for the challenging condition of ISR and we look forward to offering U.S. physicians the opportunity to treat their patients with this novel device,” Lance Bates, president of interventional cardiology therapies for Boston Scientific, said in a statement.
Thibault said the device represents a $500 million global market opportunity for Boston Scientific, and the company thinks that expansion opportunities beyond its initial indication could represent a potential $1 billion market.
The Agent study showed the technology was superior to uncoated balloon angioplasty at one year for the primary endpoint of target lesion failure, representing a 38% relative risk reduction, according to the BTIG note.
Boston Scientific said it plans to launch the balloon in the U.S. in the coming months. The device is already available in Europe, parts of Asia Pacific and Latin America to treat patients with in-stent restenosis and previously untreated small vessel coronary disease.