Dive Brief:
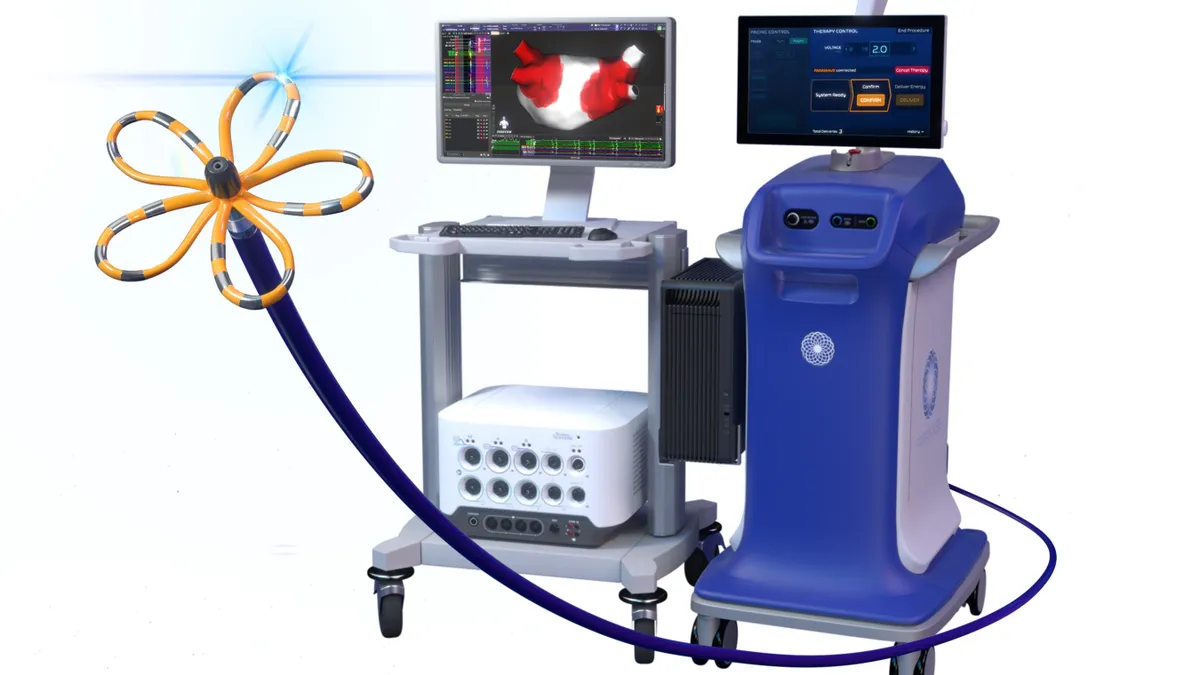
- Boston Scientific received Food and Drug Administration approval for Farawave Nav, a treatment for paroxysmal atrial fibrillation (AFib) that enables cardiac mapping and pulsed field ablation (PFA) therapy with a single integrated catheter.
- In tandem, the company gained 510(k) clearance for new software, called Faraview, to provide visualization for cardiac ablation procedures with its Farapulse PFA system, the medical device maker said Friday. Boston Scientific will immediately launch the Farawave Nav ablation catheter and Faraview software in the U.S.
- In a race among medtech companies in the PFA space, Boston Scientific is now the first with mapping-integrated PFA, “a meaningful technology step-forward,” Stifel analyst Rick Wise said Sunday in a note to clients.
Dive Insight:
Boston Scientific’s Farapulse system won FDA approval in January, shortly after Medtronic secured the first FDA approval for its PFA ablation device, Pulseselect.
PFA is a new treatment for AFib, a common abnormal heart rhythm that can increase the risk of stroke. There has been strong adoption among physicians thanks to data showing the technique offers a safer option than older treatments.
PFA is a nonthermal technique that uses short high-voltage pulses to disrupt cell membranes. Older ablation methods use either heat or extreme cold that creates small scars to block irregular heartbeats.
Boston Scientific’s Farawave Nav ablation catheter adds magnetic navigation capabilities. The Faraview software gives physicians a dynamic view of catheter placement, shape and rotation in mapped procedures with the Farapulse system, the company said. Physicians can see where pulsed fields have been applied and visualize cumulative therapy delivery to guide ablation strategy.
The technologies are exclusively compatible with Boston Scientific's cardiac mapping offerings, the company said.
Stifel’s Wise said most of Boston Scientific’s Farapulse cases currently use competitors’ mapping tools, adding that Boston Scientific can now offer “one-stop-shopping” with the Farawave catheter and an integrated mapping system.
With electrophysiology competitors also expecting mapping-integrated PFA device approvals, Boston Scientific’s authorizations allow the company to stay ahead of peers, wrote Wise.