Deep Dive
Industry insights from our journalists
-
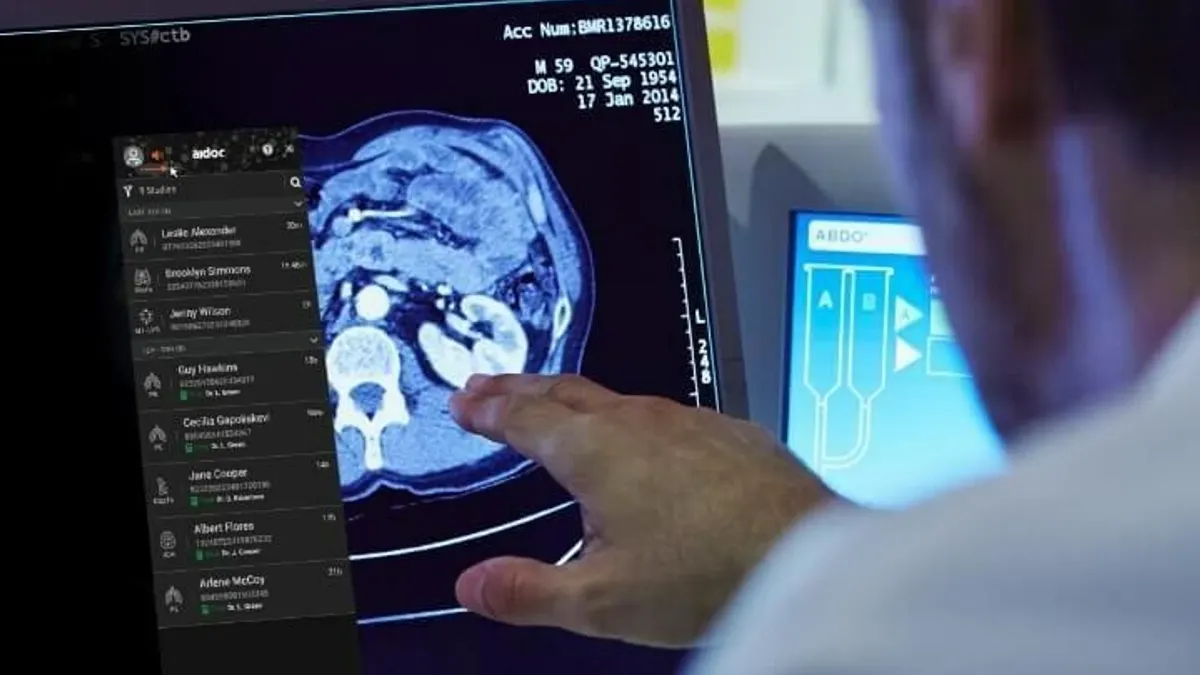
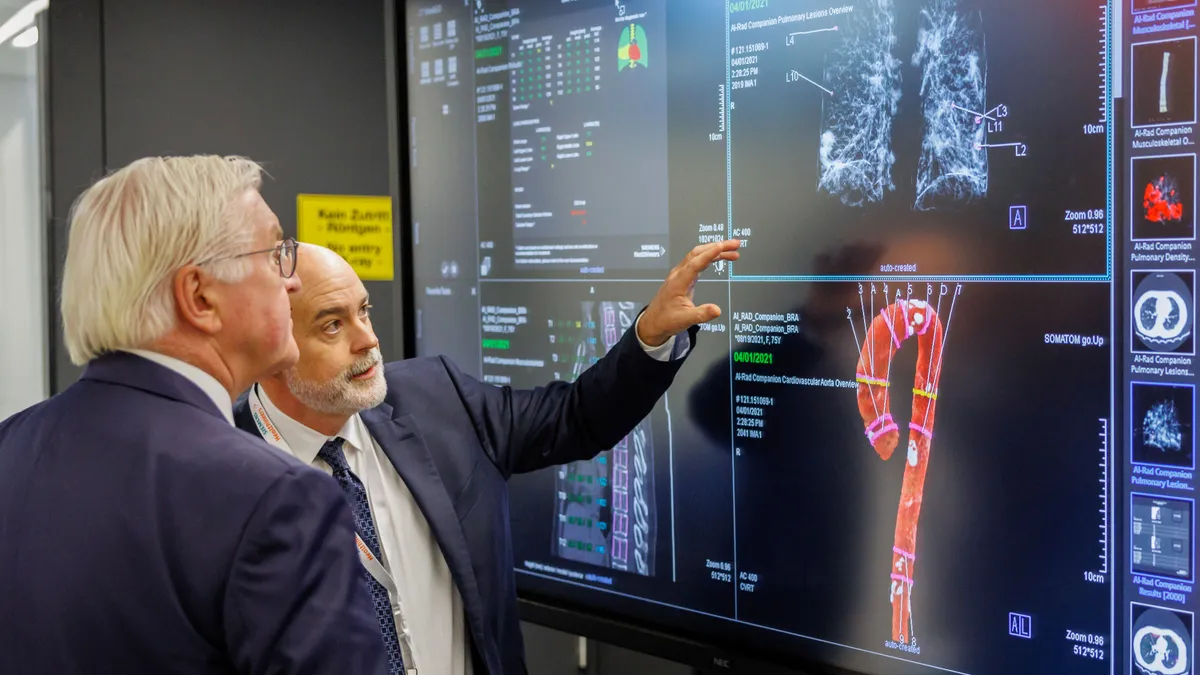
How three companies are using foundation models in radiology
Some firms, such as Aidoc, are working directly with the FDA. Others, such as HOPPR, are offering their foundation models for medtech companies to build their own AI tools.
Elise Reuter • Sept. 16, 2025 -
Foundation models are medtech’s newest trend. But what are they?
The AI models, which are built on massive datasets and can be adapted to multiple tasks, have become a part of medtech companies’ lexicon in the past few years.
Elise Reuter • Sept. 15, 2025 -
White House data sharing plan boasts big ambitions, but has scant details
Improving health data exchange is a worthy goal, but the initiative has to overcome challenges like data security, under-resourced providers and slow technology uptake, experts say.
Emily Olsen • Aug. 27, 2025 -
4 medtech topics to watch for the rest of 2025
From M&A to MDUFA and the developing market in renal denervation, MedTech Dive covers the key issues to watch in the final months of the year.
Ricky Zipp, Susan Kelly and Elise Reuter • Aug. 25, 2025 -
House committees advance reconciliation text with big impacts on healthcare
Republicans in Energy and Commerce and Ways and Means have approved their portions of President Donald Trump’s “big, beautiful bill” after marathon markups. Here’s an extensive look at what the legislation means for healthcare.
Rebecca Pifer • May 16, 2025 -
HHS layoffs may be illegal, legal experts say
The federal health department sidestepped normal procedures as it laid off 10,000 employees, according to sources. One union has already filed an internal complaint, while at least two law firms are exploring suits.
Rebecca Pifer • April 21, 2025 -
A judge blocked the FDA’s plan to regulate LDTs. What now?
One attorney said the ruling “isn’t quite the stake in the heart.”
Susan Kelly • April 16, 2025 -
5 tips for building a predetermined change control plan
The new framework is intended to make postmarket changes easier for products. Experts recommend having a clear roadmap and contacting regulators early.
Elise Reuter • March 25, 2025 -
Healthcare tackles AI oversight with no aid from Trump administration
The president’s deregulatory bent means more responsibility rests on the shoulders of healthcare stakeholders to get artificial intelligence right.
Rebecca Pifer • March 14, 2025 -
4 robotic surgery trends to watch in 2025
Intuitive Surgical will face competition this year from Medtronic and smaller companies like CMR Surgical and Moon Surgical coming to the U.S. market with robotic surgery systems.
Susan Kelly • Jan. 29, 2025 -
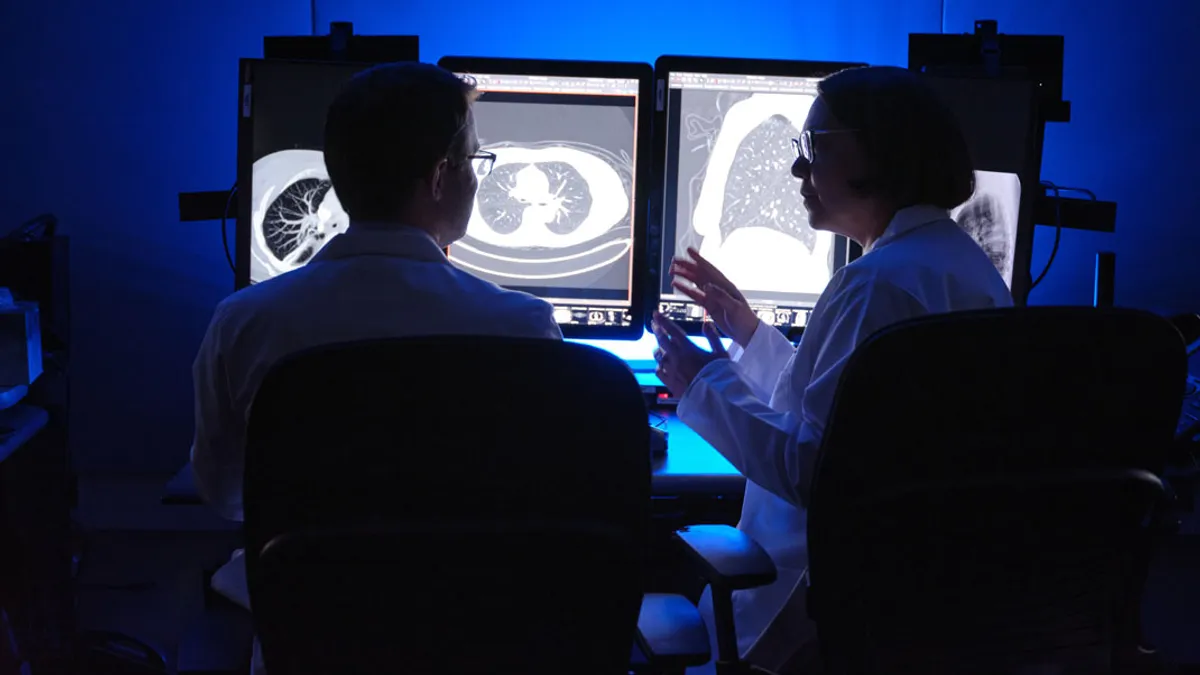
 Retrieved from Mass General Brigham on January 22, 2025
Retrieved from Mass General Brigham on January 22, 2025
AI in medtech is taking off. Here are 4 trends to watch in 2025.
New documents clarify how the FDA plans to regulate AI-enabled devices, experts say, but several important questions remain around insurance coverage and generative AI.
Elise Reuter • Jan. 22, 2025 -
Top healthcare technology trends in 2025
How the incoming Trump administration will regulate AI this year remains unclear. Meanwhile, experts say healthcare companies will continue bolstering cyber defenses to withstand increasing attacks.
Emily Olsen • Jan. 17, 2025 -
5 medtech trends to watch in 2025
After a busy 2024, experts called out competition in soft tissue robotics, uncertainty from a Trump White House and continued success for pulsed field ablation as trends to watch this year.
Ricky Zipp • Jan. 9, 2025 -
Tech companies pitch AI platforms for healthcare, outline plans for responsible rollout
Google, Microsoft and GE Healthcare say they’ll power the next wave of AI advancement. But executives are split on how to offer the tools to providers responsibly — and whether all clinicians are equally ready for them.
Susanna Vogel • Nov. 1, 2024 -
AI could be a game changer, but healthcare needs to be ‘exceedingly careful’
Artificial intelligence tools could help solve workforce challenges. Implementation, however, can be difficult, pushing organizations to consider less risky administrative and back-office tasks first.
Emily Olsen • Oct. 29, 2024 -
5 steps to navigate the FDA’s new lab developed test rule
Laboratories face a series of upcoming deadlines to comply with stricter FDA oversight of in-house tests. Here are five strategies labs can take to be ready.
Susan Kelly • Oct. 21, 2024 -
The number of AI medical devices has spiked in the past decade
The FDA has authorized nearly 1,000 medical devices with artificial intelligence features as companies including GE Healthcare and Siemens Healthineers build out their AI efforts.
Elise Reuter and Jasmine Ye Han • Oct. 9, 2024 -
4 steps to minimize the threat of legacy medical devices
Older medical devices with unsupported software pose cybersecurity threats that regulators and industry are struggling to solve. Here are four steps experts say can help mitigate risks.
Ricky Zipp • Sept. 23, 2024 -
Medtech firms have cut more than 14,000 jobs in the past 18 months
Diagnostics is the largest industry represented in the data, with more than 5,000 affected positions, according to MedTech Dive’s analysis.
Jasmine Ye Han, Ricky Zipp and Elise Reuter • Updated July 25, 2024 -
FDA’s lab developed test rule could be first check on agency’s power post-Chevron
The Supreme Court’s decision to overturn the Chevron doctrine would make it easier to challenge agency regulations, such as the LDT final rule.
Susan Kelly and Elise Reuter • July 11, 2024 -
EtO causes cancer. Device sterilizers are scrambling to find alternatives.
No one solution can match the scope and scale of ethylene oxide, but a “multi-pronged approach” can help reduce emissions, an FDA official said.
Elise Reuter • May 6, 2024 -
As cyberattacks on healthcare persist, can the FDA’s new device regs hold up?
Revamped regulations to thwart hackers are a big step forward, but issues such as legacy devices and reliance on software patches pose lingering challenges.
Ricky Zipp • April 3, 2024 -
6 ways the FDA can improve medical device recalls
Experts said improving how adverse events are tracked and requiring manufacturers to use electronic notifications could make devices safer.
Elise Reuter • March 18, 2024 -
Why Cigna is capping cost increases for pricey GLP-1 weight loss drugs
The first-of-its-kind move comes as pharmacy benefit managers continue to try to prove their value to clients, and shows how major players are shoring up to meet sky-high GLP-1 demand.
Rebecca Pifer • March 11, 2024 -
4 heart device trends shaping the medtech sector in 2024
Medtronic, Boston Scientific and J&J are among the medtech companies advancing treatments in cardiac care for when medicines are not enough.
Susan Kelly • Jan. 30, 2024