Page 2
-
BD expands Class I recall to cover 15 more Alaris pump infusion sets
BD, which has not received any complaints related to the issue, previously discontinued the devices affected by the expanded recall.
-
Deep Dive
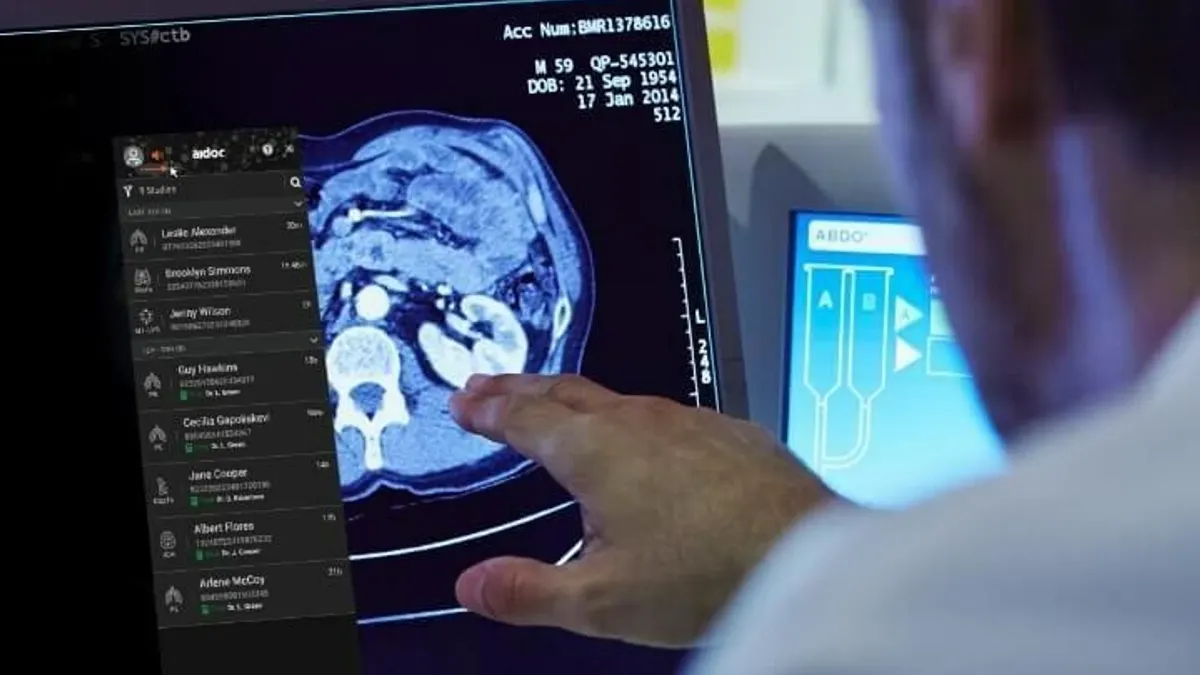
How three companies are using foundation models in radiology
Some firms, such as Aidoc, are working directly with the FDA. Others, such as HOPPR, are offering their foundation models for medtech companies to build their own AI tools.
-

 Retrieved from Insulet on September 16, 2025
Retrieved from Insulet on September 16, 2025
Insulet names new CFO; Dexcom CEO on leave
Flavia Pease will take over as Insulet’s CFO on Sept. 30. Elsewhere, outgoing Dexcom CEO Kevin Sayer is taking a medical leave of absence.
-
GOP bill extends telehealth flexibilities, sidesteps ACA subsidies
The continuing resolution would fund the government, and preserve the flexibilities, through Nov. 21. But the bill makes no mention of extending more generous financial assistance for people who buy plans on the exchanges.
-
Deep Dive
Foundation models are medtech’s newest trend. But what are they?
The AI models, which are built on massive datasets and can be adapted to multiple tasks, have become a part of medtech companies’ lexicon in the past few years.
-
Intuitive da Vinci 5 updates offer feedback during surgery
Three new software features for Intuitive’s most advanced soft tissue surgical robot have received 510(k) clearance from the Food and Drug Administration.
-
A key CDC panel meets this week to discuss vaccines. Here’s what to know.
A two-day meeting could prove an inflection point in HHS Secretary’s Kennedy’s efforts to overhaul U.S. vaccine policy, as hand-picked advisers are set to vote on guidelines for measles, hepatitis B and COVID shots.
Updated Sept. 15, 2025 -
B. Braun buys True Digital Surgery for microscopy technology
The acquisition gives B. Braun control of a company that provides technology for its Aeos robotic digital microscope.
-
New bill would reform lab test payment rates
The bipartisan legislation, introduced this week in the House and Senate, seeks to prevent more cuts to Medicare reimbursement rates for diagnostic tests.
-
Patients call for more medical device user fee funding, FDA staffing
Commenters to the FDA also advocated for safety reforms and for industry funds to be used for post-market monitoring.
-
AI drives medtech investment in 2025
Medical device companies working with artificial intelligence raised funding and were acquisition targets in recent months.
To find more content, use the "Topics" in the menu above.