Clinical Trials: Page 3
-
J&J starts trial of pulsed field ablation catheter with mapping feedback
Omnypulse expands J&J’s portfolio of investigational devices with a catheter that gathers contact force data.
By Nick Paul Taylor • Sept. 19, 2023 -
FDA releases draft guidance for studying weight loss devices
The documents arrive amid questions about whether new medicines will cut demand for weight loss procedures.
By Nick Paul Taylor • Sept. 19, 2023 -
Beacon Biosignals receives FDA clearance for sleep tracking headband
The company’s at-home device, the Dreem 3S, has six EEG electrodes to capture brain activity.
By Nick Paul Taylor • Sept. 18, 2023 -
FDA finalizes combination product guidance 7 years after sharing draft
AdvaMed called overlapping human factor requirements of the draft guidance “overly burdensome.”
By Nick Paul Taylor • Sept. 13, 2023 -
FDA proposes 3 guidances to improve 510(k) clearance process
The agency has made recommendations for selecting predicate devices, using clinical data and conducting performance testing for implants.
By Nick Paul Taylor • Sept. 7, 2023 -
Synchron brain-computer interface implanted in first 6 US patients
The device is intended to give people with severe paralysis the ability to use their thoughts to perform everyday functions, such as online communications, hands-free.
By Susan Kelly • Sept. 6, 2023 -
Guardant abandons cancer blood test trial early, restates confidence in product line
Guardant stopped enrolling new patients in the study because of its design and use of an older generation test, analysts said.
By Nick Paul Taylor • Sept. 6, 2023 -
Abbott study shows benefits of OCT imaging in stent placement
Optical coherence tomography improved doctors’ ability to place stents, but the study did not meet its primary goal for reducing a composite of deaths, heart attacks and repeat procedures.
By Susan Kelly • Aug. 31, 2023 -
Boston Scientific’s pulsed field ablation device matches standard of care in AFib trial
RBC analysts forecast “conversion of 80% to 90% of the market over a 2-3 year period” after seeing the Farapulse data.
By Nick Paul Taylor • Aug. 28, 2023 -
Edwards software linked to improved blood pressure control during surgery
Results of a registry analysis suggest “software monitoring may help reduce the duration and severity of intraoperative hypotension.”
By Nick Paul Taylor • Aug. 17, 2023 -
Boston Scientific’s pivotal pulsed field ablation trial expected to succeed: analysts
Positive results could position Boston Scientific to win U.S. approval for Farapulse in the second half of next year.
By Nick Paul Taylor • Aug. 14, 2023 -
Device volumes to hold up against threat of Wegovy, other GLP-1 drugs: analysts
Issues such as “nontrivial side effects, cost concerns and patient persistence” will mean physicians continue to use medical devices, analysts say.
By Nick Paul Taylor • Aug. 9, 2023 -
Penumbra says stroke device delayed at least 12 months due to FDA request
The setback stalls “a meaningful new product catalyst,” but the growth of Penumbra’s other devices could cushion the impact, analysts said.
By Nick Paul Taylor • Aug. 4, 2023 -
AI reduces radiologist workload in mammography clinical trial but expert urges caution
The trial linked the AI-supported screen-reading procedure, ScreenPoint Medical’s Transpara, to a 44% reduction in radiologist workload.
By Nick Paul Taylor • Aug. 2, 2023 -
FDA shares draft guidance on studying medtech treatments for opioid use disorder
The FDA aims to address challenges including inaccurate self-reported drug use, missing data and the need to show the durability of the treatment.
By Nick Paul Taylor • July 28, 2023 -
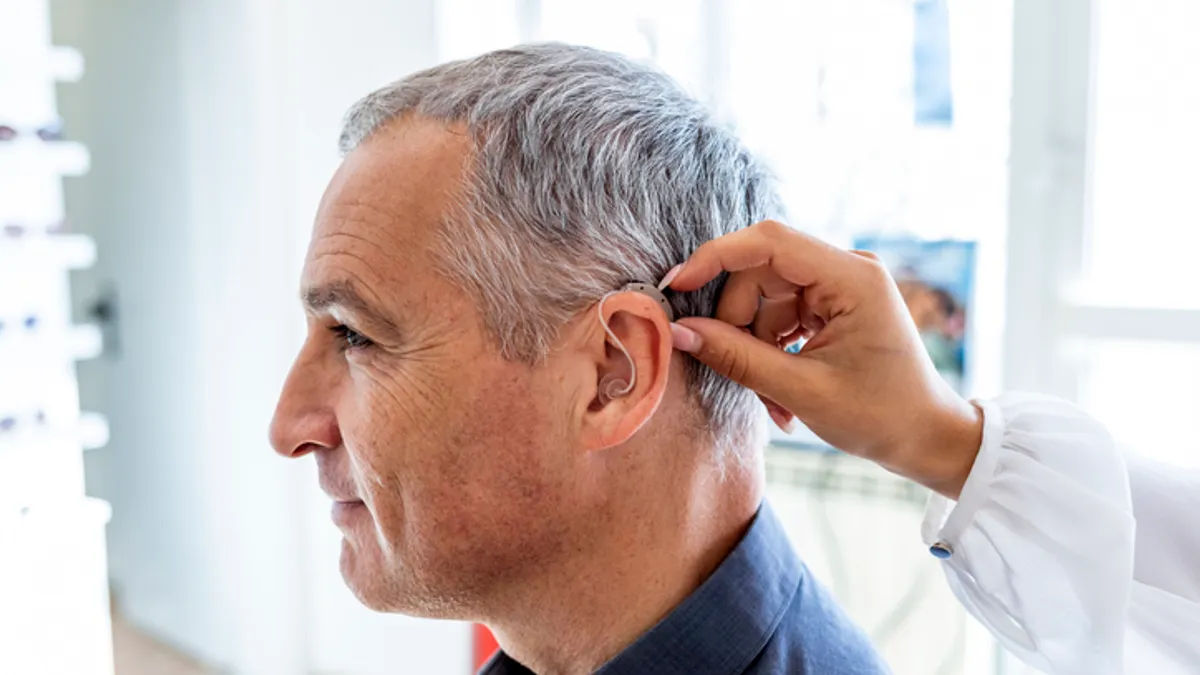
Hearing aids fail to improve cognition in randomized trial, but subgroup analysis offers hope
While there were no positive effects observed in healthy older adults, a smaller analysis pointed to a difference in those at higher risk of cognitive decline.
By Nick Paul Taylor • July 18, 2023 -

 Retrieved from Istock.
Retrieved from Istock.
FDA seeks feedback on technologies that can enable healthcare at home
The FDA is asking the medtech industry for input on how the agency can support development of devices for use in non-clinical care settings and by diverse patient populations.
By Nick Paul Taylor • June 29, 2023 -
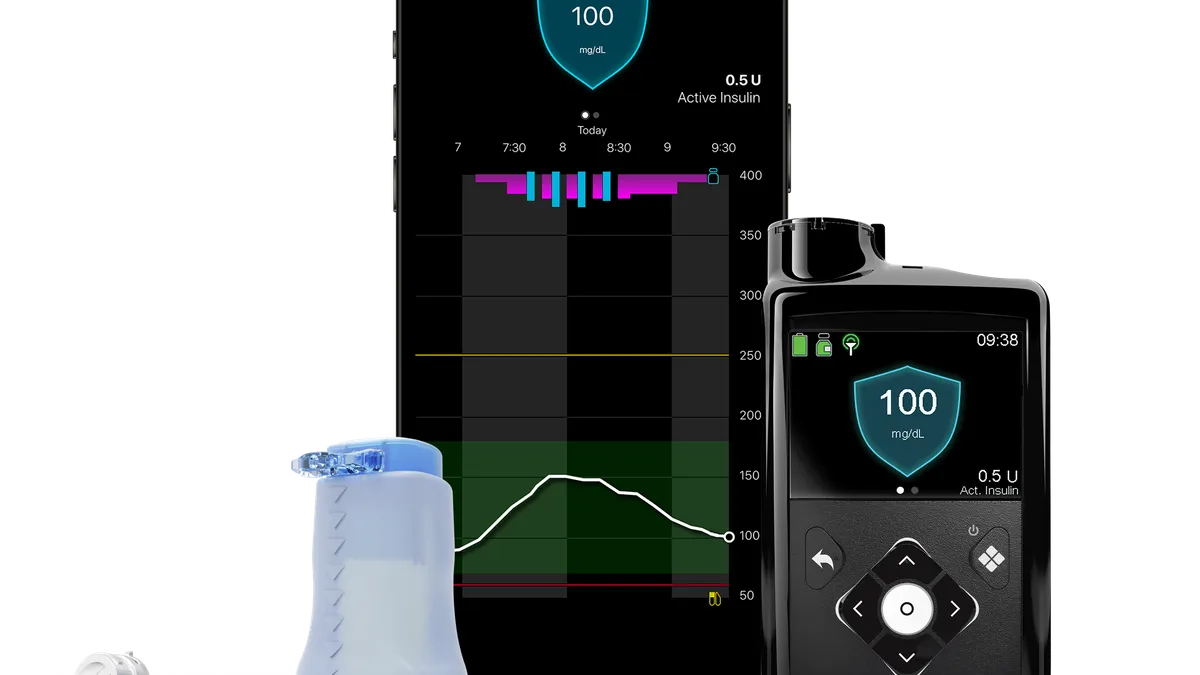
Medtronic links 780G insulin pump to benefits in kids as it works to grow sales
A clinical trial found adolescent users of the pump can achieve improved blood glucose control without precisely counting carbohydrates.
By Nick Paul Taylor • June 26, 2023 -
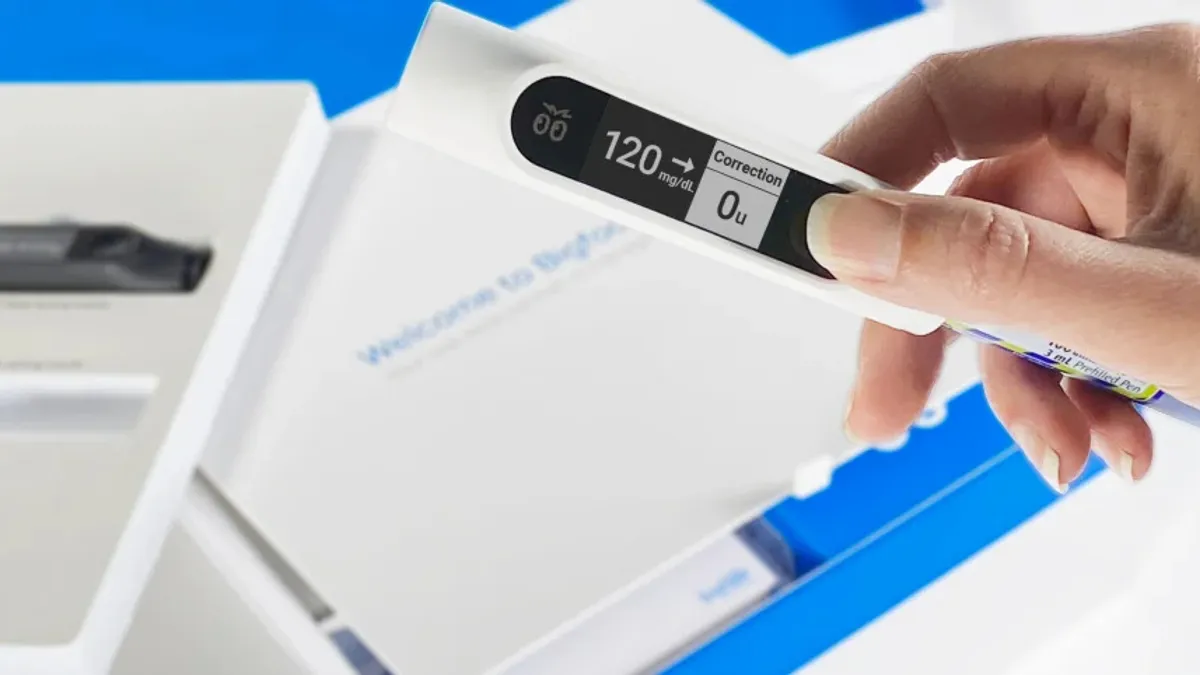
Bigfoot links smart insulin pen cap to improved glycemic control in real-world study
Using the system can bring “rapid and durable improvement” in glycemic control for older adults with Type 2 diabetes who use multiple daily injections, researchers found.
By Nick Paul Taylor • June 22, 2023 -
Exact Sciences’ next-gen colorectal cancer test hits primary goals in pivotal study
The analysis suggests the new stool-based test provides more true positives and fewer false positives than its predecessor.
By Nick Paul Taylor • June 21, 2023 -
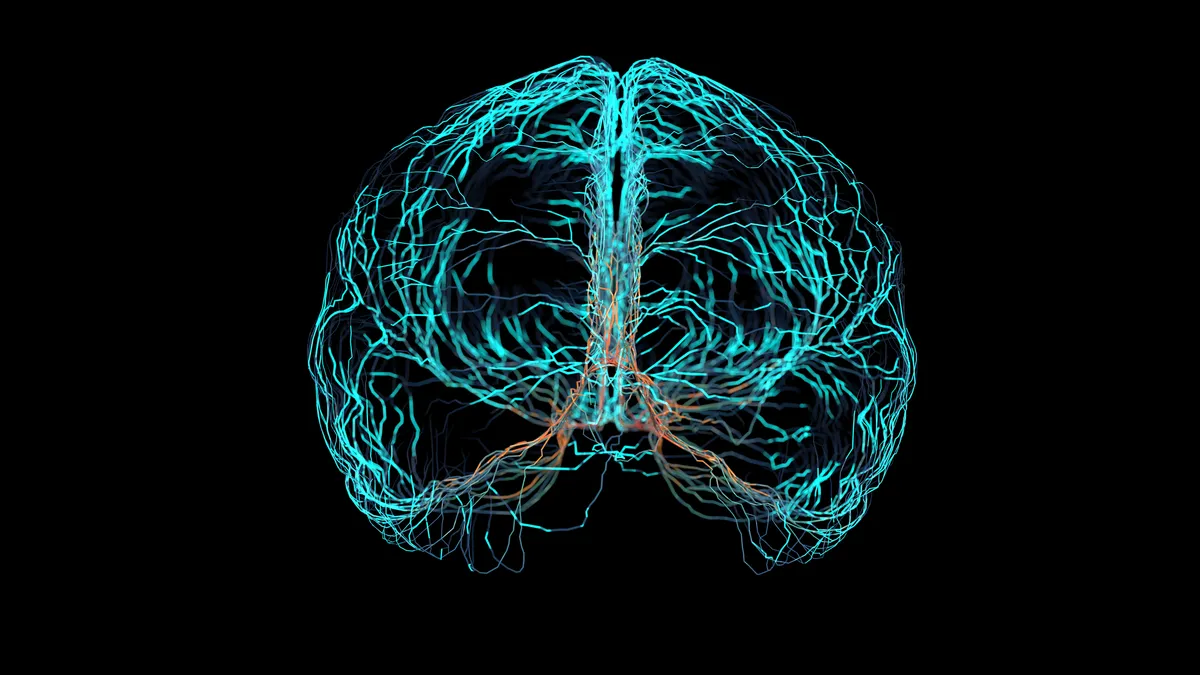
Precision Neuroscience’s brain implant maps electrical activity in a first-in-human trial
Created by a founder of Elon Musk’s Neuralink, Precision has developed a device with some 1,000 electrodes in a one square centimeter flexible film. The trial could open the way to a computer-brain interface.
By Nick Paul Taylor • June 8, 2023 -
Cue Health wins first non-emergency authorization for COVID test, days after Goldman downgrade
The FDA’s marketing authorization for Cue’s COVID-19 molecular test could boost consumer access, but the company faces strong competition from more established diagnostic firms.
By Peter Green • June 7, 2023 -
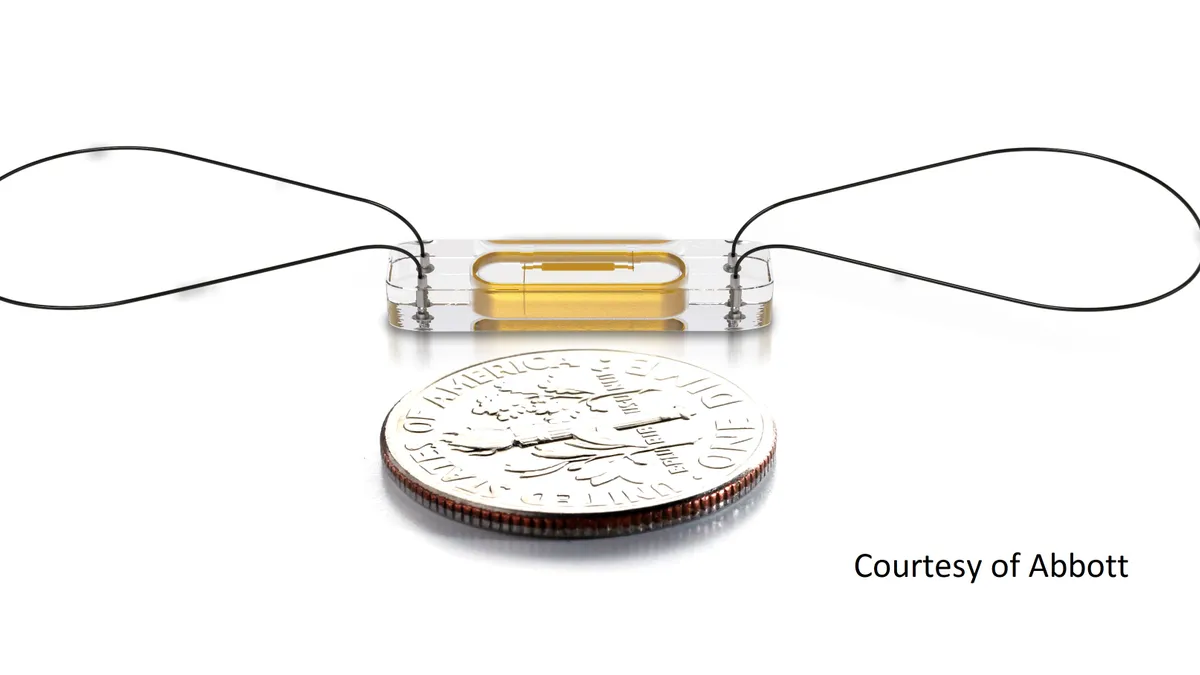
Abbott’s remote heart failure monitoring system reduces hospitalizations in clinical trial
The study is the third randomized, controlled trial globally to link CardioMEMS to a significant health benefit.
By Nick Paul Taylor • May 26, 2023 -
PulseAI algorithm beats Apple Watch software at detecting atrial arrhythmias
In a head-to-head comparison with Apple’s pre-installed software for interpreting ECG data, Pulse AI’s algorithm was better at detecting atrial arrhythmias.
By Nick Paul Taylor • May 23, 2023 -
Abbott, Boston Scientific, Medtronic share data on heart rhythm devices at conference
A series of recent innovations in pacemaker technology has opened up new markets and set the stage for increased competition.
By Nick Paul Taylor • May 22, 2023